OPERATOR’S MANUAL
Manufacturer: EU Authorized Representative:
Alcon Laboratories, Inc. Alcon Laboratories (U.K.) Ltd.
6201 South Freeway Boundary Way, Hemel Hempstead
Fort Worth, Texas 76134-2099 Hertfordshire, HP2 7UD England
U.S.A.
Produced By:
Alcon Laboratories, Inc.
15800 Alton Parkway
Irvine, California 92618-3818
U.S.A.
Telephone: 949/753-1393
800/832-7827
FAX: 949/753-6614
8065751025 Rev. P3, CATALOG NUMBER
905-2120-001 Rev. P3.1, TEXT ONLY © 2007 Alcon, Inc.
ii 8065751025
Constellation® Vision System
Constellation® Operator’s
8065751025
MANUAL REVISION RECORD
DATE REVISION ECN NUMBER AND DESCRIPTION
* Registered U.S. Patent & Trademark Office
9/15/06 P1
7/2/07 P2 (no PECN)
9/1807 P3
8065751025 iii
Constellation® Vision System
TABLE OF CONTENTS
TOPIC PAGE #
Manual Revision Record . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ii
Foreword . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . vi
Important Notice . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . vii
SECTION ONE — GENERAL INFORMATION
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-1
System Installation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.7
Source Air Pressure Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.7
Accessory Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.7
Settings Restoration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.7
Environmental Issues . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.8
User Information — Environmental Considerations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.8
Universal Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.9
EMC Statement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.9
FCC and IC Compliance Statement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.12
EU . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.12
Australia and New Zealand . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.12
Cautions and Warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.13
Diathermy, Cautery, Coagulation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.18
Product Service . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.20
Limited Warranty . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.21
SECTION TWO — DESCRIPTION
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.1
Front Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.2
Rear Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.5
Footswitch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.7
Remote Control . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.8
Front Panel Displays And Touchscreen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.12
Global Controls . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.37
System State Area . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.41
Surgery Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.58
Surgical Modes And Submodes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.65
End Case . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.94
Probes and Handpieces . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.98
The Constellation Cassette . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.106
Consumable Pak Configurations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.107
Combined Procedure Pak . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.107
Posterior Procedure Pak . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.108
Anterior Procedure Pak . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.108
SECTION THREE — OPERATING INSTRUCTIONS
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-1
Power Up Sequence . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.1
Initial System Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.2
Constellation™ Procedure Pak . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.3
Procedure Pak and Cassette Setup Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.4
Posterior Segment Setup Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.5
Anterior Segment Setup Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.6
Auto Gas Filler Setup Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.8
Fragmentation Setup Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.9
Viscous Fluid Control Setup Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.10
Extrusion Setup Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.12
iv 8065751025
Constellation® Vision System
Fiber Optic Illuminator Setup Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.12
Diathermy Setup Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.12
Pneumatic Handpiece Setup Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3.13
SECTION FOUR — CARE AND MAINTENANCE
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-1
Care and Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4.1
Upon Completion Of The Day’s Surgery Schedule . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4.2
Sterilization Instructions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4.3
Reusable Handpiece and Accessories Cleaning and Sterilization Instruction . . . . . . . . . . . . . . . . . . . . . . . 4.4
Disposal of Xenon Lamps . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4.8
SECTION FIVE — TROUBLESHOOTING
Equipment Malfunction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5.1
System Error Messages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5.2
Advisory Messages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5.3
Information Messages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5.3
SECTION SIX — ACCESSORIES AND PARTS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6.1
SECTION SEVEN — INDEX . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-1
TABLE OF CONTENTS
TOPIC PAGE #
8065751025 v
Constellation® Vision System
FIGURE # TITLE PAGE #
LIST OF ILLUSTRATIONS
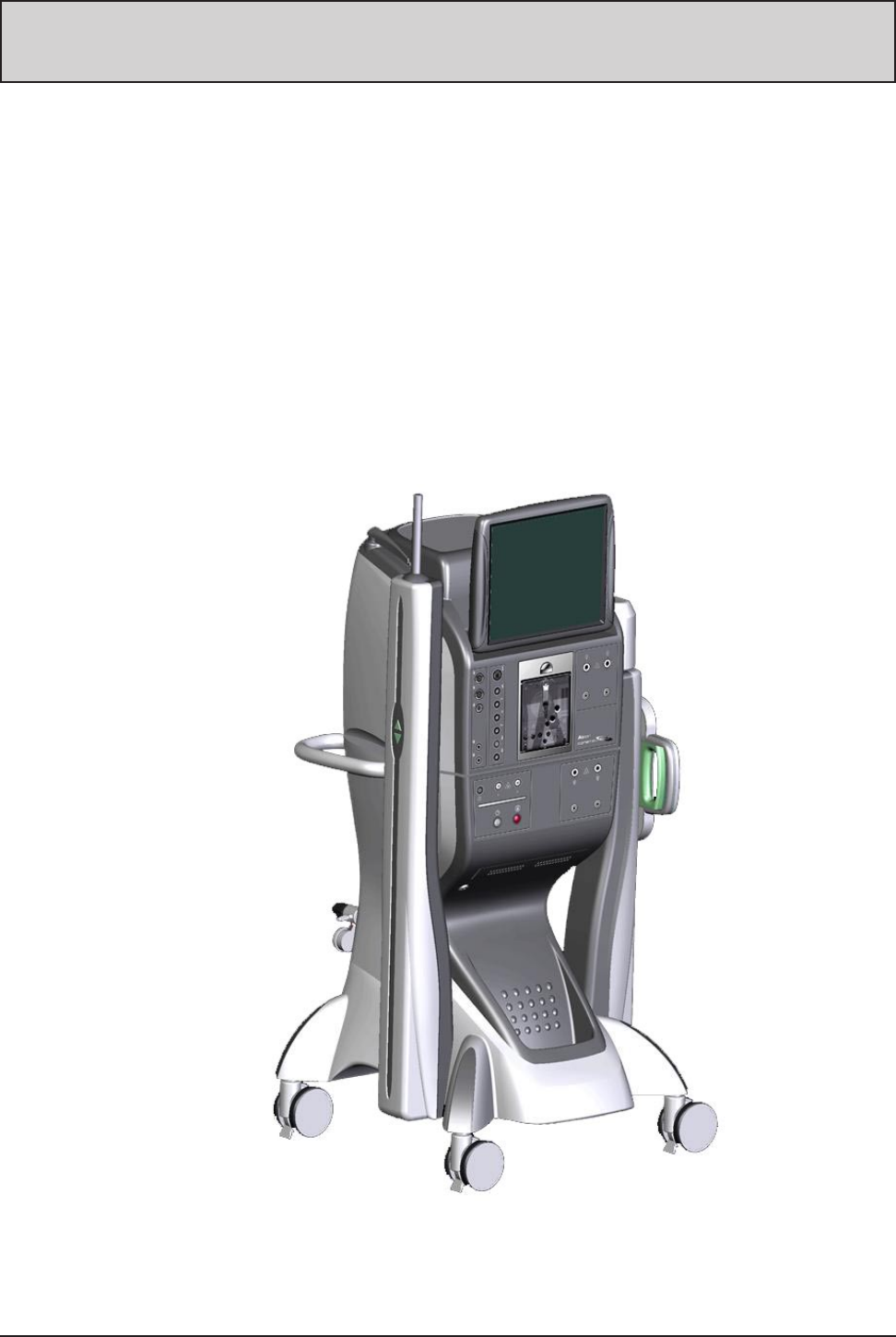
Figure 1-1 The Constellation® Vision System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1.1
Figure 1-2 Icons used with the Constellation® Vision System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1.5
Figure 1-3 Labeling used on the Constellation® Vision System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1.6
Figure 1-4 Diathermy Power Through 75 Ohm Load . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1.19
Figure 1-5 Diathermy Power Vs. Load Impedance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1.19
Figure 1-6 Diathermy Output Voltage Vs. Output Control Setting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1.19
Figure 2-1 The Constellation® Vision System Console . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.2
Figure 2-2 The Constellation® Vision System Rear Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.5
Figure 2-3 The Constellation® Vision System Footswitch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.7
Figure 2-4 The Constellation® Vision System Remote Control . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.8
Figure 2-5 Remote Control Battery Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.10
Figure 2-6 Remote Control Channel Selection Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.11
Figure 2-7 The Startup Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.12
Figure 2-8 The Main Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.13
Figure 2-9 The Main Screen Menu Bar . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.14
Figure 2-9a Procedure Modify Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.16
Figure 2-10 The Doctor Settings Popup — General Tab . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.19
Figure 2-10a The Doctor Settings Popup — Surgery/Inf/Irr Tab . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.20
Figure 2-10b The Doctor Settings Popup — Surgery/Reflux Tab . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.21
Figure 2-10c The Doctor Settings Popup — Surgery/General Tab . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.22
Figure 2-11 Doctor Settings — Footswitch Buttons Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.23
Figure 2-12 Footswitch Action Selection Popup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.24
Figure 2-13 Doctor Settings — Footswitch Treadle Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.25
Figure 2-14 Doctor Settings — Sound Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.26
Figure 2-15 Doctor Settings — Laser Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.27
Figure 2-16 Deleted . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.27
Figure 2-17 System Settings — Settings Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.28
Figure 2-18 System Settings — Connections Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.29
Figure 2-19 System Settings — VideoOverlay Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.30
Figure 2-20 Auto Gas Fill Popup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.31
Figure 2-21 View/Copy/Delete Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.32
Figure 2-22 Sample View of a Doctor Settings Report . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.34
Figure 2-23 Event Log . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.35
Figure 2-24 About Constelllation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.35
Figure 2-25 Infusion Global Control and Advanced Setup Popup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.37
Figure 2-26 F/AX Global Control and Advanced Setup Popup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.38
Figure 2-27 Irrigation Global Control and Advanced Setup Popup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.39
Figure 2-28 Diathermy Global Control and Advanced Setup Popup . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.39
Figure 2-29 Illuminator Global Control and Advanced Setup Popup . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.40
Figure 2-30 The Setup Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.41
Figure 2-31 The Fluidics Popup Screen(s) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.42
Figure 2-32 The Probe Setup Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.43
Figure 2-33 The Handpiece Setup Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.44
Figure 2-34 The Accessory Setup Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.45
Figure 2-35 The Illuminator Setup Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.46
Figure 2-36 The Laser Setup Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.47
Figure 2-37 The Fluidics Setup Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.49
Figure 2-38 The Detailed Probe Setup Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.50
Figure 2-39 The Detailed Handpiece Setup Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.51
Figure 2-40 The Detailed Accessory Setup Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.52
Figure 2-41 The Detailed Illuminator Setup Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.53
vi 8065751025
Constellation® Vision System
Figure 2-41a The Detailed Laser Setup Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.54
Figure 2-42 Video Help Popups . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.55
Figure 2-43 Prime & Test Status Bar . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.56
Figure 2-44 Consumables Popup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.56
Figure 2-45 Timer Configuration Popup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.57
Figure 2-46 The Surgery Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.58
Figure 2-47 Vacuum Control and Settings Popup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.59
Figure 2-48 Surgical Control Option . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.60
Figure 2-49 Surgical Control with Dropdown List . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.61
Figure 2-50 Accurus® Classic Surgery Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.63
Figure 2-51 Surgical Step Panel — Custom Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.63
Figure 2-51a Surgical Step Panel Scroll Buttons . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.64
Figure 2-52 Surgery Screen: Vitrectomy Mode-3D Submode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.66
Figure 2-53 Surgery Screen: Vitrectomy Mode-Momentary Submode . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.68
Figure 2-54 Surgery Screen: Vitrectomy Mode-PropVac Submode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.69
Figure 2-55 Surgery Screen: Vitrectomy Mode-VitWet Submode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.70
Figure 2-56 Surgery Screen: Phaco Mode — Burst Submode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.72
Figure 2-57 Surgery Screen: Phaco Mode — Custom Submode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.74
Figure 2-58 Surgery Screen: Phaco Mode — Pulsed Submode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.76
Figure 2-59 Surgery Screen: Phaco Mode — Continuous Submode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.78
Figure 2-60 Surgery Screen: Fragmentation Mode — Fixed Submode . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.79
Figure 2-61 Surgery Screen: Fragmentation Mode — Linear Submode . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.81
Figure 2-62 Surgery Screen: Fragmentation Mode — Momentary Submode . . . . . . . . . . . . . . . . . . . . . . . .2.82
Figure 2-63 Surgery Screen: Irrigation/Aspiration Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.83
Figure 2-64 Surgery Screen: Extrusion Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.85
Figure 2-65 Surgery Screen: Laser Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.87
Figure 2-66 Surgery Screen: Forceps Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.89
Figure 2-67 Surgery Screen: Scissors Mode — MultiCut . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.90
Figure 2-68 Surgery Screen: Scissors Mode — Proportional . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.91
Figure 2-69 Surgery Screen: VFC Mode — Extract Submode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.92
Figure 2-70 Surgery Screen: VFC Mode — Injection Submode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.93
Figure 2-71 End Case Screen: Anterior Tab . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.94
Figure 2-72 End Case: Setup Form Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.95
Figure 2-73 UltraVit Probes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.98
Figure 2-74 Pneumatic Scissors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.98
Figure 2-75 Fragmentation Handpiece . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.99
Figure 2-76 Infiniti™* Ultrasonic (U/S) Handpiece . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.99
Figure 2-77 TurboSonics® Tips . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.101
Figure 2-78 Infiniti™* U/S Handpiece shown with Infusion Sleeve and Bubble Suppression Insert . . .2.102
Figure 2-79 Ultraflow™* IT handpiece and tips . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.104
Figure 2-80 Ultraflow™* IT handpiece with infusion sleeve, reusable I/A tip, and threaded tip adapter 2.104
Figure 2-81 Ultraflow™* O-ring tool with large and small O-rings . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.104
Figure 2-82 Ultraflow™* SP handpiece (handpiece shown with .3 mm 45° tip) . . . . . . . . . . . . . . . . . . .2.104
Figure 2-83 Single use bipolar brush . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.105
Figure 2-84 The Constellation® Combined Cassette . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.106
Figure 4-1. Auto Wash: Handpiece with «Y» Adapter Connected . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4.6
Figure 4-2. Auto Wash: «Y» Adapter Connected to Injector Nozzel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4.6
Figure 4-3. Auto Wash: Unused Injectors Shown Plugged Off . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4.6
Figure 4-4. Depressurizing the Xenon Lamp . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4.8
Figure 5-1 System Fault Display Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5.1
Figure 5-2 System Error Dialog Box . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5.2
Figure 5-3 Advisory Dialog Box . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5.3
Figure 5-4 System Information Dialog Box . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5.3
8065751025 vii
Constellation® Vision System
LIST OF TABLES
Table 1-1 Technical Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1.2
Table 1-2 Terms and Abbreviations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1.4
Table 1-3 Guidance and Manufacturer’s Declaration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1.10
Table 1-4 Recommended Separation Distances Between Portable and Mobile RF
Communications Equipment and the Constellation® Vision System . . . . . . . . . . . . . . . . . . .1.11
Table 2-1. Surgical Modes And Submodes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2.66
Table 6-1. Constellation® Vision System Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6.1
TABLE # TITLE PAGE #
viii 8065751025
Constellation® Vision System
FOREWORD
This Operator’s Manual is designed to acquaint the operator and operating room personnel with
the Constellation® Vision System. The manual presents an organized summary of the operating
principles, main components, safety features, and instructions for care and use of the instrument.
The information in this manual should be supplemented with reference works on laser theory and the
interaction of laser energy with biologic tissues. No attempt is made in this manual to answer all the
questions that arise during the use of the instrument in medical procedures.
Questions concerning technique, safety and effectiveness should be referred to pertinent publications
or recognized medical experts in laser surgery. Physicians should not attempt to treat patients with
this instrument if not thoroughly familiar with its operation, or if in doubt as to its safe operation. All
personnel authorized to use this instrument should be required to be thoroughly familiar with this
manual.
Please contact Alcon for complete technical support and service if you have questions concerning any
aspect of this instrument’s operation or if it fails to perform satisfactorily.
To order supplies in U.S.A.:
800-862-5266
FAX: 800-241-0677
Outside U.S.A.: Contact your local Alcon representative for supplies.
8065751025 ix
Constellation® Vision System
IMPORTANT NOTICE
Equipment improvement is an on-going process and, as such, changes may be made to the equipment
after this manual is printed.
Pay close attention to WARNINGS and CAUTIONS in this manual. WARNINGS are written to
protect individuals from bodily harm. CAUTIONS are written to protect the instrument from damage.
Illustrations contained in this manual are for reference only.
It is recommended that maintenance be performed by a qualified Alcon Field Engineer.
Alcon Surgical shall not be liable for any damage resulting from failure to comply with the enclosed
instructions.
Alcon reserves the right to change specifications without further notice.
Operator Profile
The Constellation Vision System is designed to be operated by two basic groups; surgeons and
nurses/scrub techs. The surgeon focus is primarily constrained to the footswitch and display panel.
The design of the footswitch allows the surgeon to map any function to any switch position,
assuming the function is valid in a particular scenario. The display screen was designed to mount
on an articulating arm to allow optimum placement of the display so the surgeon can reference it at
any time. The design also incorporated items specifically for nurses and scrub techs, who routinely
control the machine via the front panel and remote control. The design incorporates color coding on
all connectors and tubing to facilitate easy identification of the ports. In addition, the graphical user
interface closely resembles controls commonly found on web sites, which this operator profile is
expected to be highly proficient at using.
CAUTION
U.S. Federal Law restricts this device to sale by or on the order of a physician only.
WARNINGS!
For systems containing the optional laser module: Use of controls or adjustments, or
performance of procedures other than those specified herein may result in hazardous
laser radiation exposure.
A qualified technician must perform a visual inspection of the following components
every twelve months. In case of a deficiency, do not use the system; call Alcon Technical
Services.
— Warning Labels
— Power Cord
— Fuses
A qualified technician must check ground continuity and both polarities for leakage
current every twelve months to ensure they are within the applicable standard (for
example: EN 60601-1/IEC 601-1). Values must be recorded, and if they are above the
applicable standard, or 50% above your first measurement, do not use the system; call
Alcon Technical Services.
Use of accessories and cables other than those provided may result in increased
emissions or decreased immunity of the system. Portable and mobile RF
communications equipment can affect this medical electrical equipment.
x 8065751025
Constellation® Vision System
LAST PAGE OF THIS SECTION
Comments or corrections concerning this manual should be addressed to:
Alcon
Technical Services Group
PO BOX 19587
Irvine, CA, USA 92623
All rights reserved. No part of this manual may be reproduced, transmitted, or stored in a retrieval
system, in any form or by any means; photocopying, electronic, mechanical, recording, or otherwise;
without prior written permission from Alcon Laboratories, Inc.
8065751025 1.1
Constellation® Vision System
SECTION ONE
GENERAL INFORMATION
Introduction
The Constellation® Vision System is a multifunctional surgical tool for use in anterior
and posterior segment ophthalmic surgeries. The product’s capabilities include
driving a variety of handpieces that provide the ability to cut vitreous and tissues,
emulsify the lens, illuminate the posterior segment of the eye, and apply diathermy to
stop bleeding. Vacuum is used to remove ocular matter from the eye and is provided
by connecting tubing from the handpiece to a port on the fluidics cassette. Irrigation/
infusion capability is provided to replace fluid in the eye, and enters the eye directly
via either an infusion cannula or flows through a handpiece. The graphical operator
interface is menu driven. The operator provides inputs using the touchscreen panel,
the remote control, voice commands, and the footswitch.
Figure 1-1 The Constellation® Vision System — The Constellation® Vision System is
a multifunctional surgical tool is used in anterior and posterior segment
ophthalmic surgeries.
1.2 8065751025
Constellation® Vision System
CONSOLE
DIMENSIONS:
Tabletop:
Height: 160.0 cm (63.0 inches)
Width: 76.2 cm (30.0 inches)
Depth: 77.5 cm (30.5 inches)
Base:
Height: 63.5 cm (25.0 inches)
Width: 53.3 cm (21.0 inches)
Depth: 57.2 cm (22.5 inches)
WEIGHT:
Tabletop: 61.2 kg (135 pounds)
Base: 72.6 kg (160 pounds)
ENVIRONMENTAL LIMITATIONS:
Operating Non-Operating
Altitude: -125 to 2000 m -125 to 3000 m
(-410 to 6562 feet) (-410 to 9843 feet)
Temperature: 10° C to 35° C -10 to 55°C
(50° F to 95° F) (14° F to 131° F)
Relative Humidity: 10% to 95% 10% to 95%
without without
condensation condensation
ELECTRICAL REQUIREMENTS: The console accepts the following
ranges or input commercial power voltages and frequencies and meets
the leakage currents specified in IEC 60601-1. Protection against electrical
shock is Class I.
100-120 Vac 50/60 Hz 12 A max. Fuse: T 10 A / 250 V slow blow
220-240 Vac 50/60 Hz 7.5 A max. Fuse: T 5 A / 250 V slow blow
FOOTSWITCH
DIMENSIONS:
Height: 14.0 cm (5.50 inches)
Width: 22.9 cm (9.00 inches)
Depth: 43.2 cm (17.0 inches)
WEIGHT: 5.4 kg (12 pounds)
ENVIRONMENTAL: The footswitch construction is water tight in
compliance with IEC 60601-1 and IEC 60601-2-2, subclause 44.6 aa.
ELECTRICAL: The footswitch is connected to the console via electrical
cable.
All power and communications enter/exit the footswitch from this
cable.
Table 1-1 CONSTELLATION® VISION SYSTEM SPECIFICATIONS — This table is a quick reference point to
identify system specifications, system requirements, and performance figures.
PERFORMANCE SPECIFICATIONS
PRESSURIZED INFUSION/IRRIGATION @SEA LEVEL:
Range: 0 to 120 mmHg
Accuracy: ±(2% of setpoint +5 mmHg)
Flow Rate: 0 — 20 cc/min. for infusion (20 Ga)
0 — 60 cc/min. for irrigation
Setpoint Transient: 500 ms maximum
IOP CONTROLLED INFUSION:
Setpoint Range: 0-120 mmHg
Repeatability1: ± 2 mmHg2
Setpoint Response Time: <500 ms (20 Ga)
Transient Disturbance
Response Time: <500 ms3
Flow Range: 0-20 cc/min
1 BSS Dual chamber mode.
2 BSS medium, 20 gauge high flow Cannula, steady state condition
at rated flow range
3 Transient condition from no flow state to 10cc/min
ASPIRATION/SUCTION @SEA LEVEL:
Standard & Reduced
Pressure Range: 0-650 mmHg Vacuum
Minimal Pressure Range: 0-600 mmHg Vacuum
Pressure Accuracy: ±(2% of Setpoint +5 mmHg)
Flow Range:
Posterior Modalities: 0-20 cc/min
Anterior Modalities: 0-60 cc/min
Transient Response Time
(Standard Pressure Range): From– 0 to -400 mmHg @0 cc/min
10-90% Rise Time:300 msec max
90-10% Fall Time: 300 msec max
IRRIGATION/ASPIRATION @SEA LEVEL:
Sub Modes: Cap Vac, I/A Max
Vacuum:
Standard & Reduced
Pressure Range: 0 to -650 mmHg
Minimal Pressure Range: 0 to -600 mmHg Vacuum
Vacuum Accuracy: 2% of displayed value + 5
mmHg
Flow Rate: 10 cc/min minimum + 1.5cc at
50 mmHg vacuum max
VACUUM @ SEA LEVEL:
Vitrectomy: 0 to 650 mmHg
Fragmentation: 0 to 650 mmHg
Extrusion: 0 to 650 mmHg
Extraction: 0 to 650 mmHg
Irrigation/Aspiration: 0 to 650 mmHg
Phacoemulsification: 0 to 650 mmHg
8065751025 1.3
Constellation® Vision System
PERFORMANCE SPECIFICATIONS…continued
LOW PRESSURE AIR SOURCE (LPAS) @SEA LEVEL:
Pressure Range: 0 – 120 mmHg at rated flow
Pressure Accuracy: ±3% of Setpoint +3 mmHg
Flow Rate: 1.2 slpm minimum at 120 mmHg
VITRECTOMY:
Submodes: 3D, Momentary, PropVac, VitWet
Cut Rate:
UltraVit™ 5000 Probe: 100 to 5000 cpm
UltraVit™ 2500 Probe: 100 to 2500 cpm
DIATHERMY:
Frequency: 1.5 Mhz ± 10%.
Waveshape: Sinusoidal
Output power 10 Watts maximum at 100% setting
with 75 ± 10% ohm non-inductive load
Power range 0 — 100% of maximum output power
ILLUMINATION:
Lumen Accuracy: ±30%
Light Output through 20GA 0.5
NA Fiber Probe: 0-200 hrs: 50 lumens minimum
at 100% setting
201-400 hrs:25 lumens minimum
at 100% setting
Light Output through
25GA 0.63 NA Fiber Probe: 0-200 hrs: 15 lumens minimum
at 100% setting
201-400 hrs:10 lumens minimum
at 100% setting
FRAGMENTATION:
Submodes: Linear, Fixed, Momentary
Tip Stroke @ 100%: 3.1 ± 0.5 mils at 100% power
Resonant Frequency: 39.0 ± 1.9 KHz
Pulse Rate Range: 0 – 100 pps
SCISSORS:
Submodes: Proportional, Multi-Cut
Proportional Pressure: 0-50 psi @sea level
Multi Cut Rate: single cut to 450 cpm
PROPORTIONAL AND CONTINUOUS REFLUX @SEA LEVEL:
Pressure Range: 0 to 120 mmHg
Pressure Accuracy: ±(2% of Setpoint +5 mmHg)
MICRO REFLUX:
Pressure Range: 100 ± 15% mmHg1
Volume: 50 ± 10 µL1
1 measured with unoccluded Accurus® probe and aspiration tubing
VISCOUS FLUID CONTROL:
Submodes: Inject, Dual, Extract
Injection Pressure: 0 to 551.6 KPascal (0 to 80 psi)
0 to 482.7 KPascal
@ Reduced (0 to 70 psi)
Extract Vacuum
at Sea Level:
0 to 650 mmHg
Table 1-1 CONSTELLATION® VISION SYSTEM SPECIFICATIONS…continued
AUTO-GAS FILLING (AGF):
Maximum Gas Pressure: 10 psig
Fill Purity: 99.9% gas concentration
following fill
AUTO-STOPCOCK:
Response Time: 0.5 seconds minimum
Pressure (Liquid): 0-120 mmHg
Rated Flow (Liquid): 20 cc/min
Pressure (LPAS): 0-120 mmHg
Rated Flow (LPAS): 1.2 slpm
PHACOEMULSIFICATION:
Submodes: Burst, Pulsed, Continuous
Tip Stroke @ 100%: 3.5 ± 0.5 mils
Resonant Frequency: 34khz – 42Khz ± 10%.
Pulse Rate Range: 0-100 pulses per second
Burst Length: 2.5 sec – user adjustable
Burst Pulse durations: 5ms to 500ms
ANTERIOR VITRECTOMY:
Submodes: Wet, Dry
Cut Rate: 0 to probe maximum
LASER (optional):
Treatment beam:
Class: IV
Power: 30 mW to 2 W (maximum)
Wavelength: 532 nm
Aiming beam:
Class: II
Power: less than 1mW
Wavelength: 635 nm ± 5 nm
DOCTOR MEMORIES:
Storage Capacity: xxx Doctors
Memory Cells per Dr: xx (xx anterior, xx posterior, xx common)
TIMER:
Range: 0 to xx h
Resolution: 1 s
TONE VOLUMES @ 1 Meter:
Errors/Faults/Invalid Key: 40 to 65 dB, short tones
Diathermy: 40 to 65 dB, continuous tone
Advisory/Timer Expire/Elev Infusion: 0 to 65 dB, short tones
Frag/Phaco/Vacuum: 0 to 65 dB, continuous tone
Valid Key: Factory set and not adjustable
Volume Accuracy: 6 dB
VOICE CONFIRMATION: 0 to 65 dB
REMOTE CONTROL:
Method: Infrared
Channels: 4
1.4 8065751025
Constellation® Vision System
Term or Abbreviation
BSS PLUS®
cmH2O
cpm
Detent
Diathermy
Extrusion
F/AX
Frag
Global Function
Highlighted
I/A
IEC
ISO
IV
LCD
mmHg
N/A
PEL
PIN
psi
pps
slpm
U/S
VFC
Vit
Description
Balanced Salt Solution enriched with bicarbonate, dextrose, and glutathione.
Centimeters of water.
Cuts Per Minute.
A discrete footpedal position at which more force is required to depress the footpedal to
the next position.
The production of heat in body tissues by electric current for therapeutic purposes.
A mode where vacuum is available to remove fluid/matter.
Fluid Air Exchange.
Fragmentation.
A function whose status and controls are independent of the current footpedal position
and surgery mode.
To center attention by video reversing the function key and changing the key color from
gray to blue.
Irrigation/Aspiration.
International Electromechanical Commission.
International Standards Organization.
Intravenous.
Liquid Crystal Display.
Millimeter of Mercury. A unit of vacuum.
Not Applicable.
Patient Eye Level. A difference in height between the cassette and the patient eye level.
Personal Identification Number.
Pressure per Square Inch. A unit of pressure.
Pulses Per Second.
Standard Liters Per Minute.
Ultrasound.
Viscous Fluid Control.
Vitrectomy. Extraction of the vitreous from the vitreous cavity.
Table 1-2 Terms and Abbreviations
8065751025 1.5
Constellation® Vision System
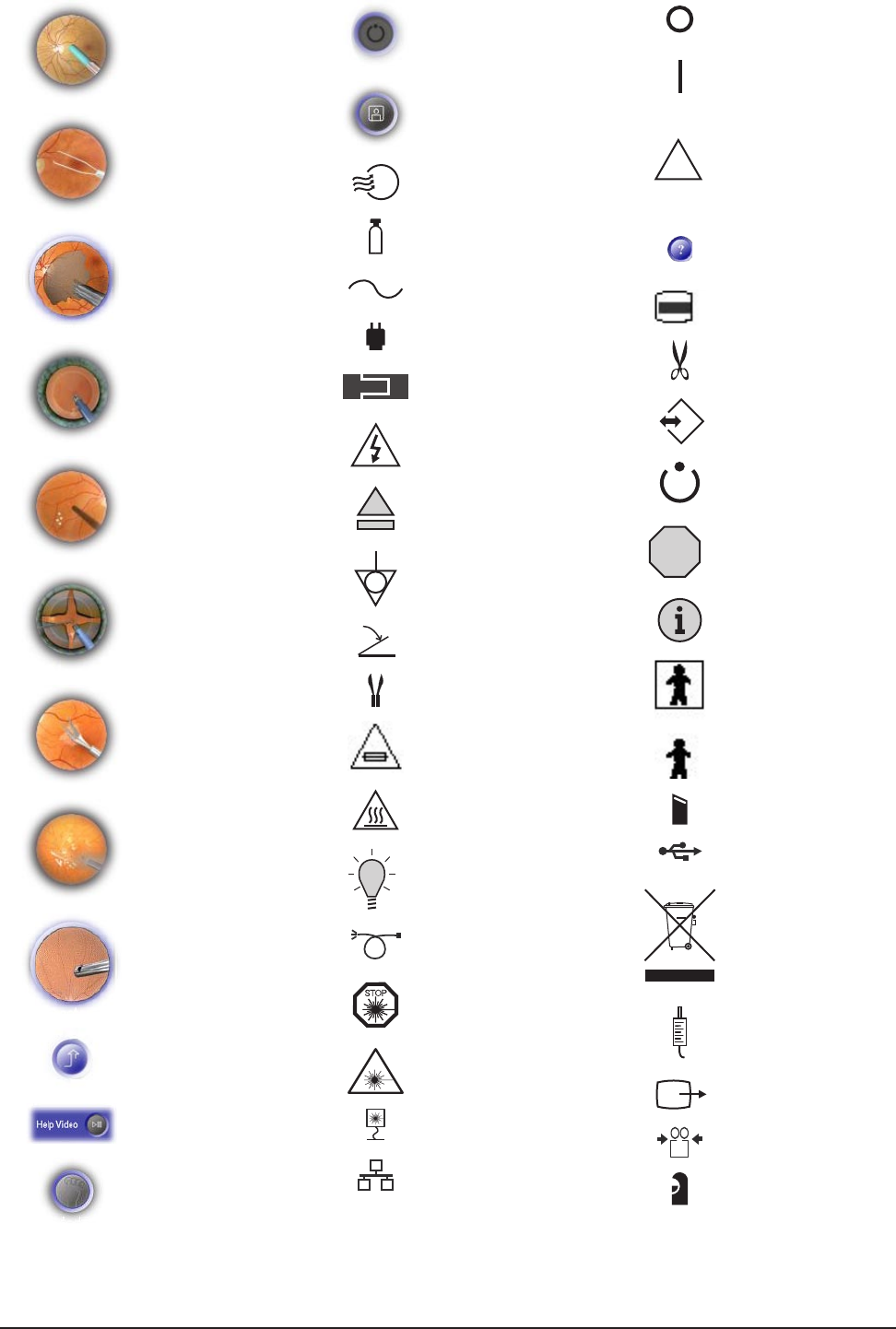
Figure 1-2 ICONS USED WITH THE Constellation® Vision System — Icons identifying modes, functions, etc., that
are used with the Constellation® Vision System are identified in this chart.
Extrusion
Forceps
Fragmentation
Irrigation/
Aspiration
Laser
Phaco
Scissors
Viscous
Fluid
Control
(VFC)
Vitrectomy
Expand
Window
Help Video
Modify
Power
Save
Air Pressure
Input
Auto Gas Filling
(AGF)
Alternating
Current
Coagulation
Connector
Connection
Indicator
Dangerous
Voltage
Eject
Equipotentiality
Footswitch
Forceps
Fuse
Hot
Illuminator
Laser
Connection
Laser
Emergency Stop
Switch
Laser Port 1
Tethered Laser
Network
Connection
Off
On
Consult
Operator’s
Manual, or
System Error or
Advisory
Opens
Operator’s
Manual
Video Recorder
Scissors
Connector
Serial In/Out
Standby State
System Fault
System
Information
Type BF
Equipment
Type B
Equipment
U/S Handpiece
Connector
USB Connector
Use appropriate
take-back
system (see
Environmental
Considerations in
this manual).
Viscous
Fluid Control
Connector
VGA Out
Video In/Out
Vitreous Cutter
Connection
1.6 8065751025
Constellation® Vision System
Figure 1-3 LABELING USED ON THE CONSTELLATION® VISION SYSTEM — Labels used on the Constellation®
Vision System console are identified and illustrated here.
LASER 532 nm — 2W — CW
DIODE LASER 635nm — 1mW
CLASS 4 LASER PRODUCT
IEC 60601-2-22: 1995
RAYONNEMENT LASER
EXPOSITION DANGEREUSE
DE LÔEIL OU DE LA PEAU AU
RAYONNEMENT DIRECT OU DIFFUS
APPAREIL Å LASER DE CLASSE 4
LASER RADIATION
AVOID EYE OR SKIN EXPOSURE TO
DIRECT OR SCATTERED RADIATION
CLASS 4 LASER PRODUCT
Laser 532nm — 2W
DIODE LASER 635nm — 1mW
RISQUE D’EXPLOSION SI UTILISATION
EN PRESENCE D’ANESTHETIQUE INFLAMMABLE
POSSIBLE EXPLOSION HAZARD IF USED IN
THE PRESENCE OF FLAMMABLE ANESTHETIC
DANGER
USE OF THIS LASER WITHOUT A SAFETY FILTER
MAY RESULT IN DAMAGE TO THE OPERATOR’S EYES
L’USAGE DE CE LASER SANS FILTRE MEDECIN
PEUT ENDOMAGER LES YEUX DE L’UTILISATEUR
WARNING
Visible laser radiation.
Avoid eye or skin exposure to
direct or scattered radiation.
Class IV laser product
Labels for gas containers
8065751025 1.7
Constellation® Vision System
System Installation
In the USA contact the Alcon Technical Services Department for uncrating and
installation at (800) 832-7827. Outside the USA contact your local Alcon affiliate.
Source Air Pressure Requirements
The Constellation® Vision System
is designed to operate using different levels of
source air pressure. The system operates automatically with pressures of 58 (4 bar) to
120 psi (8.3 bar). Between 4 bar and 5 bar, vacuum performance is reduced. Between
4 bar and 5.5 bar, VFC inject performance is reduced.
Accessory equipment connected to or used with this equipment must be certified
according to the respective IEC Standard (e.g., IEC 60950 for data processing
equipment, and IEC 60601-1 for medical equipment). The Constellation® is shipped
with English and metric air fittings compliant with EN739:1998. Anyone connecting
additional equipment or otherwise causing a different system configuration than
provided by Alcon is responsible for continued compliance to the requirements
of System Standard IEC 60601-1-1. If in doubt, consult the Technical Services
department or your local Alcon representative.
Settings Restoration
When a loss of power occurs, the Constellation® Vision System retains its current
settings and mode in memory. When power is reestablished a System Information
popup window appears asking:
«Do you want to restore the system’s previous settings and mode?»
The user can press Yes to restore the previous settings, or No to enter the default
settings. Disconnecting the power cord, or turning the system off using the rear panel
power switch, is considered a loss of power; using the rear panel standby switch is
not.
1.8 8065751025
Constellation® Vision System
Environmental Issues
Follow local governing ordinances and recycling plans regarding disposal or
recycling of device components.
WARNINGS!
Laser: There are potential hazards when inserting, steeply bending, or improperly
securing the fiberoptic. Not following the recommendations of the manufacturer
may lead to damage of the fiber or delivery system and/or harm to the patient or
user.
Since the aiming beam passes down the same delivery system as the treatment
beam, it provides a good method of checking the integrity of the delivery system.
If the aiming beam spot is not present at the distal end of the delivery system,
or its intensity is reduced or it looks diffused, this a possible indication of a
damaged or not properly working delivery system. If there is any doubt, contact
Alcon Technical Services.
The use of flammable anesthetics or oxidizing gases such as nitrous oxide (N2O)
and oxygen should be avoided. Some materials — for example cotton wool when
saturated with oxygen — may be ignited by the high temperatures produced in normal
use of the laser equipment. The solvents of adhesives and flammable solutions
used for cleaning and disinfecting should be allowed to evaporate before the laser
equipment is used. There is also danger of ignition of endogenous gases.
User Information — Environmental Considerations
The equipment that you have purchased requires the use of natural resources for its
production. This equipment may also contain hazardous substances which could have
potential effect on the environment and human health if disposed of improperly.
In order to avoid the entry of any such substances into our environment and to
promote natural resource conservation, we encourage you to use the appropriate take-
back systems. Such take-back systems reuse or recycle many of the materials in your
end-of-life equipment in a beneficial way. Please contact your local Alcon office for
assistance in take-back options through Alcon or other providers.
The crossed-bin symbol located on this equipment reminds you to use take-back
systems, while also emphasizing the requirement to collect waste equipment
separately, and not dispose of it as unsorted municipal waste.
If you need more information on the collection, reuse or recycle systems available to
you, please contact your local or regional waste administration, or contact your local
Alcon office for more information.
8065751025 1.9
Constellation® Vision System
Universal Precautions
Universal precautions shall be observed by all people who come in contact with the
instrument and/or accessories to help prevent their exposure to blood-borne pathogens
and/or other potentially infectious materials. In any circumstance, wherein the exact
status of blood or body fluids/tissues encountered are unknown, it shall be uniformly
considered potentially infectious and handled in accordance with OSHA guidelines.
EMC Statement
It is important to install and use the equipment in accordance with the instructions
in order to prevent harmful interference with other devices in the vicinity. If this
equipment causes harmful interference to other devices (determined by turning the
equipment off and on), the user is encouraged to try to correct the interference by one
or more of the following measures:
Reorient or relocate the other device(s).
• Increase the distance between the equipment.
• Connect this equipment into an outlet on a circuit different from that to which the
other device(s) is connected.
• Consult the manufacturer or your Alcon field service engineer for help.
Table 1-2 Guidance and Manufacturer’s Declaration — Electromagnetic Emissions — The Constellation®
Vision System is intended for use in the electromagnetic environment specified below. The
customer or the user of the system should assure that it is used in such an environment.
Emissions Test Compliance Electromagnetic Environment-Guidance
RF emissions
CISPR 11
Group 1 The Constellation® Vision System uses RF energy only for its internal function.
Therefore, its RF emissions are very low and are not likely to cause any interference
in nearby electronic equipment.
RF emissions
CISPR 11
Class A Based on extensive field experience the Constellation® Vision System is suitable
for use in all establishments other than domestic and those directly connected
to the public low voltage power supply network that supplies buildings used for
domestic purposes.
The EMC Statement provides guidance on steps to take in case of electromagnetic
interference.
Harmonic emissions
IEC 61000-3-2
Class A
Voltage fluctuations/
Flicker emissions
IEC 61000-3-3
Complies
1.10 8065751025
Constellation® Vision System
Table 1-3 Guidance and Manufacturer’s Declaration — Electromagnetic Immunity — The Constellation®
Vision System is intended for use in the electromagnetic environment specified below. The
customer or the user of the Constellation® Vision System should assure that it is used in
such an environment.
Immunity Test IEC 60601 Test Level Compliance Level Electromagnetic Environment-Guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
• ±6 kV contact
• ±8 kV air • ±6 kV contact
• ±8 kV air
Floors should be wood, concrete, or ceramic tile. Do
not use around floors that are covered with synthetic
material to avoid laser stoppage due to ESD.
Electrical fast
transient/burst
IEC 61000-4-4
• ±2 kV for power
supply lines
• ±1 kV for input/output
lines
• ±2 kV for power
supply lines
• ±1 kV for input/
output lines
Mains power quality should be that of a typical
commercial or hospital environment. To avoid laser
stoppage due to fast transients avoid powering the
Constellation® Vision System on the same branch
circuit with sources that can generate fast transients
(inductive switching; e.g., high current motors).
Surge
IEC 61000-4-5 • ±1 kV differential
mode
• ±2 kV common mode
• ±1 kV differential
mode
• ±2 kV common
mode
Mains power quality should be that of a typical
commercial or hospital environment. To avoid
laser stoppage due to power-line surges consider
powering the Constellation® Vision System through
branch circuit that has surge suppressor for
protection against lightning surges (e.g., at power
panel to surgical/office suite).
Voltage dips, short
interruptions, and
voltage variations
on power supply
input lines
IEC 61000-4-11
• <5% UT (>95% dip in
UT) for 0.5 cycle
• 40% UT (60% dip in
UT) for 5 cycles
• 70% (30% dip in UT)
for 25 cycles
• <5% (>95% dip in
UT) for 5 sec
• <5% UT (>95% dip
in UT) for 0.5 cycle
• 40% UT (60% dip
in UT) for 5 cycles
• 70% (30% dip in
UT) for 25 cycles
• <5% (>95% dip in
UT) for 5 sec
Mains power quality should be that of a typical
commercial or hospital environment. If the uses of
the Constellation® Vision System require continued
operation during power mains interruptions, it is
recommended that the Constellation® Vision System
be powered from an uninterruptible power supply
or a battery.
Power frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
3 A/m 3 A/m Power frequency magnetic fields should be at
levels characteristic of a typical location in a typical
commercial or hospital environment.
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 Vrms
150 kHz to 80 MHz
3 V/m
80 MHz to 2.5 GHz
3 Vrms
3V/m
Portable and mobile RF communications equipment
should be used no closer to any part of the
Constellation® Vision System, including cables, than
the recommended separation distance calculated
from the equation applicable to the frequency to
the transmitter.
Recommended separation distance:
d = 1.2√P
d = 1.2√P 80 MHz to 800 MHz
d = 2.3√P 800 MHz to 2.5 GHz
Constellation® Vision System
where P is the maximum output power rating to the
transmitter in watts (W) according to the transmitter
manufacturer and d is the recommended separation
distance in meters (m).
Field strength from fixed RF transmitters, as
determined by an electromagnetic site surveya,
should be less than the compliance level in each
frequency rangeb.
Interference may occur in the vicinity of
equipment marked with following symbol.
Note: UT is the a.c. mains voltage prior to application of the test level.
Note 1: At 80 MHz and 800 MHz, the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from
structures, objects, and people.
a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) and land mobile radios, amateur radio, AM and
FM radio broadcast, and TV broadcast cannot be predicted theoretically with accuracy. To access the electromagnetic environment
due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in
which the Constellation® Vision System is used exceeds the applicable RF compliance level above, the Constellation® Vision
System should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary,
such as re-orienting or relocating the Constellation® Vision System.
b Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
8065751025 1.11
Constellation® Vision System
Table 1-4 Recommended Separation Distances Between Portable and Mobile RF Communications
Equipment and the Constellation® Vision System — The Constellation® Vision System is
intended for use in an electromagnetic environment in which radiated RF disturbances are
controlled. The customer or the user of the Constellation® Vision System can help prevent
electromagnetic interference by maintaining a minimum distance between portable and
mobile RF communications equipment (transmitters) and the Constellation® Vision System
as recommended below, according to the maximum output power of the communications
equipment.
Separation distance according to frequency of transmitter
(m)
Rated maximum output
power of transmitter
(W)
150 kHz to 80 MHz
d = 1.2√P
80 MHz to 800 MHz
d = 1.2√P
800 MHz to 2.5 GHz
d = 2.3√P
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
For transmitters rates at a maximum output power not listed above, the recommended separation distance d in meters (m) can
be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter manufacturer.
Note 1 — At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
Note 2 — These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from
structures, objects, and people.
1.12 8065751025
Constellation® Vision System
USA – Federal Communications Commission (FCC) Compliance Statement
This device complies with Part 15 of the FCC Rules. Operation is subject to the
following two conditions:
This device may not cause harmful interference.
This device must accept any interference received, including interference that may
cause undesired operation.
Caution
Change or modifications made to this equipment not expressly approved by Alcon
may void the FCC authorization to operate this equipment.
FCC Radiation Exposure Statement
Caution
To ensure that the radio transmitter complies with current FCC regulations limiting
both maximum output RF power and human exposure to radio frequency radiation, a
separate distance of at least 20 cm must be maintained between the unit’s antenna and
the body of the user and any nearby persons at all times, and unit’s antenna must not
be co-located or operating in conjunction with any other antenna or transmitter.
Canada – Industry of Canada (IC) Compliance Statement
This device complies with Industry Canada Radio Standards Specification RSS-210.
Operation is subject to the following two conditions:
This device may not cause harmful interference
This device must accept any interference, including interference that may cause
undesired operation of the device.
This ISM device complies with Canadian ICES-001.
Cet appareil ISM est conforme à la norme NMB-001 du Canada.
Antenna Notices
This device has been designed to operate with the antenna having a maximum gain of
5 dBi. Antenna having a gain greater than 5 dBi is strictly prohibited for use with this
device. The required antenna impedance is 50 ohms.
To reduce potential radio interference to other users, the antenna type and its gain
should be so chosen that the equivalent isotropically radiated power (EIRP) is not
more than that required for successful communication.
Exposure of Humans to RF Fields
This device complies with the RF exposure limits for humans as called out in RSS-
102.
1.
2.
1.
2.
8065751025 1.13
Constellation® Vision System
Europe – R&TTE Directive 99/5/EC
This device complies with the requirements of the Council Directive 99/5/EC
(R&TTE).
Caution
The radio equipment is intended to be used in all EU and AFTA countries. Outdoor use
may be restricted to certain frequencies and/or may require a license for operation.
Contact local Authority for procedure to follow.
Note: Combinations of power levels and antennas resulting in a radiated power
of above 100mW equivalent isotropic radiated power (e.i.r.p) are considered as
not compliant with the above mentioned directive and are not allowed for use
within the European community and countries that have adopted the European
R&TTE directive 1999/5/EC.
For more details on legal combinations of power levels and antennas, contact Alcon
Compliance.
Equipment contains radio transmitters:
Wireless LAN device (WiFi)
Frequency or frequency band of transmission: 2.412 – 2.462 GHz
Type and frequency characteristics of the modulation: CCK, DQPSK, DBPSK,
OFDM
The Effective Radiated Power (ERP): 17 – 18dBm (50 – 64mW)
Radio Frequency Identification (RFID) device
Frequency or frequency band of transmission: 13.56 MHz
Type and frequency characteristics of the modulation: ASK
The Effective Radiated Power (ERP): -119 dBm (1260 pW)
Australia and New Zealand
This device complies with the Australian/New Zealand Standard AS/NZS 4268:
2003 Radio Equipment and Systems – Short Range Devices – Limits and methods
measurement, and AS/NZS 4771 (2000 + A1: 2003) Technical characteristics and test
conditions for data transmission equipment operating in the 900 MHz, 2.4 GHz and
5.8 GHz bands and using spread spectrum modulation techniques.
•
•
•
•
•
•
1.14 8065751025
Constellation® Vision System
Cautions and Warnings
Please contact Alcon for instrument setup and in-service training.
If you have any questions or require additional information, please contact your local
Alcon representative or the Technical Services Department. For locations outside the
USA, please contact your local authorized Alcon Service/Sales office.
• Good clinical practice dictates the testing for adequate irrigation, aspiration flow,
and operation as applicable for each handpiece prior to entering the eye.
• Do not use the Constellation® Vision System
system
near flammable anesthetics.
• Use only Alcon-supplied A.C. power cords. Prior to plugging the power cord into
its power source, ensure that the proper voltage selection has been made. See Care
and Maintenance section of this manual for instructions.
• Provide at least two feet of clearance at the rear of the unit for fan intakes and
exhausts. This ensures unrestricted air flow for adequate console cooling.
• A handle on the instrument cart is used for moving the instrument. The cart should
be pulled, not pushed, over elevator and door thresholds.
WARNING!
The Constellation® Vision System power cord is a medical grade power cord with
the least leakage current per foot rating available. Extension of the power cord
by hospital staff is not recommended. Unauthorized extension of the power cord
could result in injury.
Presurgical Setup Instructions
Presurgical setup instructions must be performed as outlined in this manual. If an
error message is displayed on the front panel, refer to Troubleshooting of this Manual.
If a problem persists, DO NOT PROCEED. Contact your local Alcon Surgical
Service Representative.
NOTE: If an inconsistency exists between the setup instructions in this manual and
the Directions For Use (DFU) supplied with a consumable pak, follow the DFU.
Vitreous Probes
Do not operate vitreous probes in air. This could result in performance degradation
and/or potential hazard.
8065751025 1.15
Constellation® Vision System
Cautions and Warnings
Ultrasonic Handpieces
Power loss may occur if handpiece tip is not securely tightened into Fragmentation
and Phaco handpieces.
WARNING!
Use of a Phaco handpiece at power settings greater than 80% continuously for
over 4 minutes can result in ultrasonic system failure. Allow the system to cool
for 8 minutes between heavy usage of this type.
If proper cleaning procedures are not performed immediately after each surgical
procedure, tissue debris and salts from irrigating solution may collect. This could
permanently damage the handpiece and could jeopardize cleanliness and/or create
biohazard conditions for the patient. Remove all debris prior to autoclaving handpiece.
CAUTION
Never ultrasonically clean the Fragmentation and Phaco handpieces; irreparable
damage will result.
WARNINGS!
Use of the phaco handpiece in the absence of irrigation flow and/or in the presence
of reduced or lost aspiration flow can cause excessive heating and potential
corneal and/or scleral burns.
Use of the Fragmentation handpiece in the absence of aspiration flow can cause
excessive heating and potential scleral burns.
Handpiece Tips
Scissors, frag, and phaco handpiece tips must be fully tightened to their handpieces. If
not secured properly, the handpieces may not operate correctly. Ensure, however, that
tips are not so tight that they cannot be removed after use. Use only Alcon supplied
fragmentation tip wrenches; otherwise, damage to tips and/or handpiece may occur.
WARNING!
For phaco surgery use only Alcon-certified Turbosonics® MicroTip™ configurations
(.9 mm). Alcon does not recommend the use of standard Turbosonics® tips (1.0
mm) with the Constellation® Vision System.
1.16 8065751025
Constellation® Vision System
Diathermy Function
To ensure safe operation of the Diathermy function, use only Alcon cables and
accessories. Diathermy performance can be guaranteed only when using Alcon
Surgical components or Alcon-endorsed components. Cables should always be
positioned in such a way that contact with the patient is prevented.
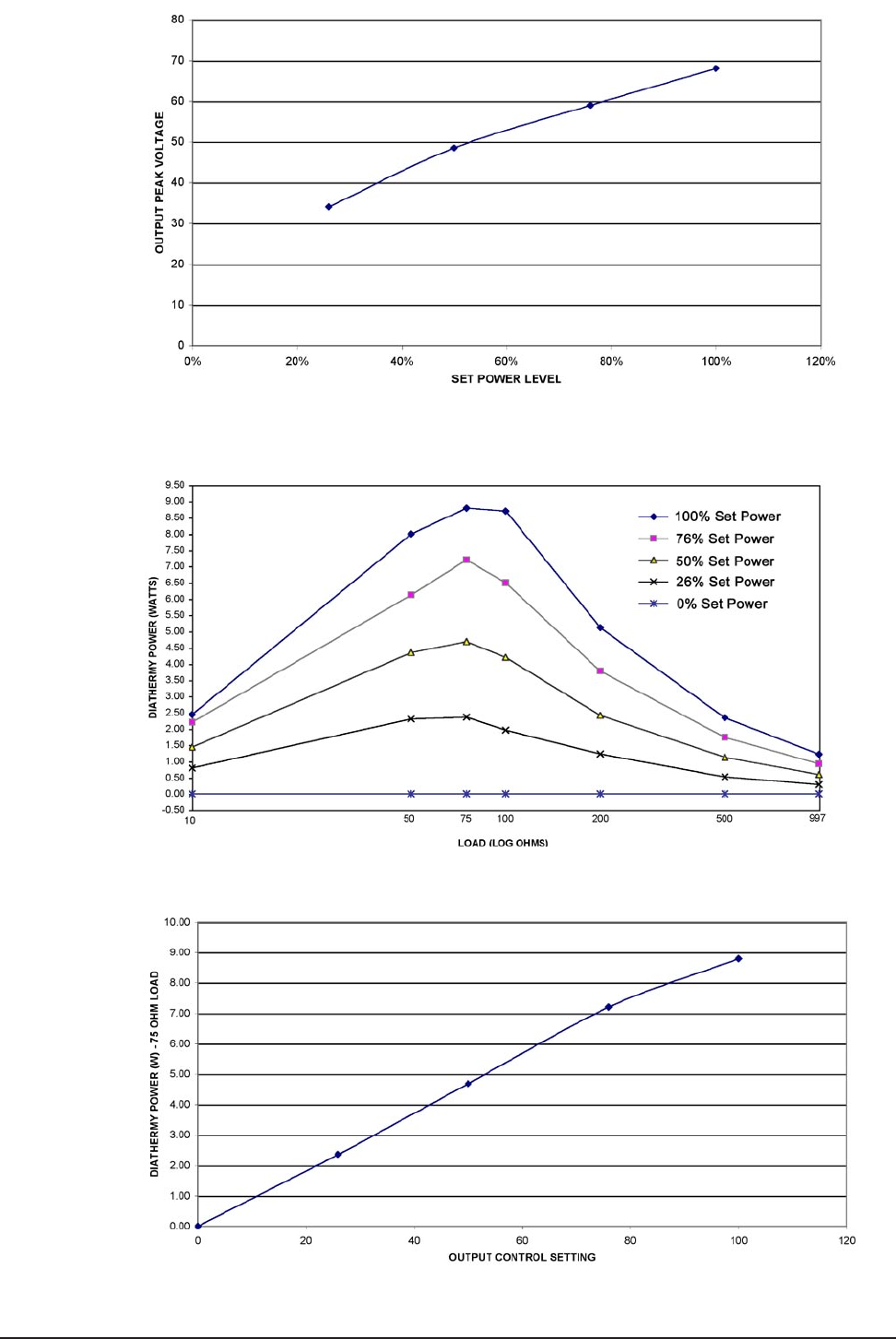
See Figures 1-4 through 1-6 for diathermy power specifications.
WARNING!
• Do not use the diathermy function on patients with pacemakers or implanted
defibrillatory devices. If electrosurgery is used on patients with implanted
cardiac pacemakers or defibrillatory devices or pacemaker electrodes, be aware
that irreparable damage to the pacemaker or defibrillatory device and its function
may occur and lead to ventricular fibrillation. Please check with the pacemaker
or defibrillatory device manufacturers for their recommendations.
• Failure of the HF surgical equipment (diathermy circuitry) could result in an
unintended increase of output power.
Listed below are general precautions to be followed when using the Diathermy function:
• To ensure safe operation of the
Diathermy
function, only approved cables and
accessories must be used (See your Alcon representative).
Diathermy
performance
can be guaranteed only when using Alcon components or Alcon-endorsed
components.
• To reduce the risk of accidental burns, caution should always be taken when
operating high-frequency surgical equipment.
• Interference produced by the operation of high-frequency surgical equipment may
adversely influence the operation of other electronic equipment.
• Accessories should be checked regularly; electrode cables should particularly be
checked for possible damage to the insulation.
• The lowest power level in
Diathermy
step should always be selected for the
intended purpose.
• Skin-to-skin contact (for example between the arms and body of the patient)
should be avoided, for example by insertion of dry gauze.
• When HF (high frequency) surgical equipment and physiological monitoring
equipment are used simultaneously on the same patient, any monitoring
electrodes should be placed as far as possible from the surgical electrodes. Needle
monitoring electrodes are not recommended.
• In all cases, monitoring systems incorporating high frequency current-limiting
devices are recommended.
• The cables to the surgical electrodes should be positioned in such a way that
contact with the patient or other leads is avoided.
• Temporarily unused active electrodes should be stored so that they are isolated
from the patient.
8065751025 1.17
Constellation® Vision System
• The use of flammable anaesthetics or oxidizing gases such as nitrous oxide (N2O)
and oxygen should be avoided if a surgical procedure is carried out in the region
of the thorax or the head, unless these agents are sucked away.
•
Non-flammable agents should be used for cleaning and disinfection wherever possible.
• Flammable agents used for cleaning or disinfecting, or as solvents of adhesives,
should be allowed to evaporate before the application of HF surgery. Some
materials, for example cotton, wool and gauze, when saturated with oxygen may
be ignited by sparks produced in normal use of the HF surgical equipment.
Illuminator Function
Housed inside Constellation® tabletop, the illuminator provides two channels of
illumination from a single xenon arc lamp. Two front panel sockets accept ACMI
compatible fiber optic light guides to provide intraocular illumination.
When installed, an optional illuminator is housed inside Constellation® base and
provides two channels of illumination from a single xenon arc lamp.
To gain access for bulb replacement or servicing, follow directions in section four of
this manual. Be aware that using the illuminator at high settings will reduce the life of
the bulbs.
WARNING!
The illuminator bulbs become extremely hot. Never handle a bulb until it has
cooled considerably from its operating temperature. Do not touch bulb directly
with fingers at any time.
The bulb of the xenon lamp is under constant high pressure. There is a risk it may
burst with explosive force if knocked or damaged. Protective measures:
— Keep the lamp in its protective sleeve at all times during installation
— If you are handling the lamp without its protective sleeve, always wear safety
goggles, a face mask, gauntlets with wrist protectors and a breast protector.
1.18 8065751025
Constellation® Vision System
Cautions and Warnings
Footswitch
Never pick up or move the footswitch by holding the cable. Damage may result.
Cassette
During initialization the drain pump is rotated to the home position; therefore, keep
hands and fingers clear of cassette well during power-on initialization. Manually
rotating the hub roller in the cassette well when power is on and a cassette is not
installed can cause incorrect cassette loading and/or can cause injury to fingers.
WARNINGS!
All fluids aspirated during surgery should be treated as biohazards. Take
appropriate precautions when handling instruments and lines in contact with
aspirated fluids.
Drain bag volume should not exceed 500cc “Max. Capacity.” Exceeding this
volume may result in a biohazardous condition.
Consumables
Do not use consumable paks beyond the expiration date stamped on the outer
packaging. Sterile consumable medical devices should not be reused (Accreditation
Manual for Hospitals, 1982); they are intended for single use only. Improper usage
or assembly could result in a potential hazardous condition for the patient. Alcon
assumes no responsibility for complications that may arise as a result of the reuse or
improper usage of consumables.
The equipment used in conjunction with Alcon Constellation® Vision System
consumables constitutes a complete system. Use of consumables other than Alcon
consumables may affect system performance and create potential hazards, and if it
is determined to have contributed to the malfunction of the equipment under service
contract, could result in the voidance of the contract and/or invoicing at prevailing
hourly rates.
WARNING!
Attach only Alcon supplied consumables to console and cassette luer fittings. Do
not connect consumables to the patient’s intravenous connections.
In all cases, the instrument setup instructions contained in this manual, and all label
instructions in the package, should be thoroughly understood prior to using any of the
Constellation® Vision System Pak configurations.
Ensure that tubing is not occluded during any phase of operation.
8065751025 1.19
Constellation® Vision System
Cautions and Warnings
Consumable Paks
If any item in a consumable pak is received in a defective condition, Alcon is to be
notified immediately. Do not use any of the contents if the sterile package is damaged
or the seal is broken in any way. Paks are identified by lot number that provides
traceability and should be given to the Customer Service Department.
Phone Alcon Customer Service At: Please Write To Alcon At:
(800) 862-5266 or Alcon
(817) 293-0450 Attn: Product Complaints
6201 South Freeway
Fort Worth, TX 76134-2099
Diathermy, Cautery, Coagulation
In the past, some of Alcon’s products have referred to the feature “Cautery” or
«Coagulation.» The Constellation® Vision System and this operator’s manual use the
word “Diathermy” based on the following definitions:
• Diathermy — introducing an electric field into a body part to produce heat.
• Cautery — cutting and burning method associated with two hot wires passing a
current between them; cutting away skin; halting bleeding.
• Coagulation — an isolated bipolar current supplied to conductors (e.g. forceps).
Current passes between these electrodes, halting bleeding.
1.20 8065751025
Constellation® Vision System
Figure 1-4 DIATHERMY POWER THROUGH 75 OHM LOAD
Figure 1-5 DIATHERMY POWER VS. LOAD IMPEDANCE
Figure 1-6 DIATHERMY OUTPUT VOLTAGE VS. OUTPUT CONTROL SETTING
Note: Maximum output peak-to-peak voltage is about 140V without resistive load.
8065751025 1.21
Constellation® Vision System
PRODUCT SERVICE
For product service, please contact Alcon’s Technical Services Department at the
number provided below.
Operators experiencing problems with the system should refer to the Operating
Instructions and Troubleshooting sections of this manual. A problem which persists
should be referred to the Alcon Technical Services Department or your local
authorized service representative.
For optimum performance, it is the user’s responsibility to schedule preventive
maintenance service on the system and its accessories one time each year. Alcon’s
Field Service Engineers are trained and equipped to provide the highest quality of
workmanship.
Safety performance should be verified by the user (e.g., qualified service personnel)
at least twice a year. Ground resistance must be under 0.1 ohms. Leakage current
must be under 500 µA.
To avoid unnecessary shipping, please contact your Alcon Technical Services
Department prior to the return of any system or accessories. If return of the equipment
is deemed necessary, a Return Material Authorization will be issued with appropriate
shipping instructions.
Alcon Laboratories, Inc.
Technical Services Department
15800 Alton Parkway
Irvine, California 92618-3818
(949) 753-1393
(800) 832-7827
1.22 8065751025
Constellation® Vision System
LIMITED WARRANTY
Alcon Laboratories, Inc., will repair or replace at its option, any system or
accompanying accessories (excluding the optical fiber) found to be defective in
material and/or workmanship for a period of one (1) year from the date of initial
installation. This warranty applies to the original purchaser of the system, when said
system is properly installed, maintained, and operated in accordance with published
instructions.
Alcon Laboratories shall not be obligated to provide services under this warranty for
damage to or destruction of systems covered where such damage or destruction is (i)
a result of or caused by fire or explosion of any origin, riot, civil commotion, aircraft,
war, or any Act of God including, but not limited to lightning, windstorm, hail,
flood, earthquake, or (ii) caused by customer’s misuse or improper servicing of said
systems.
The express warranty above is the sole warranty obligation of Alcon, and the
remedy provided above is in lieu of any and all other remedies. There are no other
agreements, guarantees, or warranties — oral or written, express or implied — including
without limitation warranty of merchantability or fitness for a particular purpose.
Alcon shall have no liability whatsoever for any incidental or consequential damages
arising out of any defect, improper use, or unauthorized service or repair.
WARNING!
The disposables used in conjunction with Alcon instrument products constitute
a complete surgical system. Use of disposables and handpieces other than those
manufactured by Alcon may affect system performance and create potential
hazards. If it is determined that disposables or handpieces not manufactured
by Alcon have contributed to the malfunction of the equipment during warranty
period, service will be provided at prevailing hourly rates.
LAST PAGE OF THIS SECTION
Регистрационное удостоверение на медицинское изделие ФСЗ 2009/04025
Внимание, документ содержит изменения:
История вносимых изменений
Фактический адрес заявителя
Юридический адрес заявителя
Фактический адрес изготовителя
Юридический адрес изготовителя
236650
Система офтальмологическая хирургическая Constellation® Vision System с принадлежностями, производства Алкон Лабораториз, Инк., США. I. Constellation Vision system. Комплектация люкс
236650
Система офтальмологическая хирургическая Constellation® Vision System с принадлежностями, производства Алкон Лабораториз, Инк., США. II. Constellation Vision system. Комплектация стандарт
236650
Система офтальмологическая хирургическая Constellation® Vision System с принадлежностями, производства Алкон Лабораториз, Инк., США. III. Constellation Vision system. Комплектация эконом
Система офтальмологическая хирургическая Constellation® Vision System с принадлежностямиI. Constellation Vision system. Комплектация люкс:
1.Консоль.
2.Лампа консоли.
3.Футляр для лампы консоли.
4.Антенна для передачи информации на принтер.
5.Руководство по эксплуатации.
6.Сканер штрих кодов.
7.Пульт дистанционного управления.
8.Держатель сканера.
9.Чехол консоли.
10.Шланг.
11.Держатель для инфузионного раствора.
12.Педаль управления.
13.Стойка-держатель.
14.Инструментальный столик.
15.Балласт.
16.Тележка-подставка.
17.Система для подключения газа.
18.Регулятор газового баллона ISPAN перфторпропан.
19.Регулятор газового баллона ISPAN серы гексафторид.
20.Чехол подставки.
21.Модуль освещения.
22.Лампа модуля освещения.
23.Футляр для лампы модуля освещения.
24.Лазер.
25.Педаль управления лазером.
26.Задняя панель для тележки-подставки.
27.Интерфейс для подключения лазера.
28.Лазерный блокиратор с ключами.
29.Инструкция по подключению лазера.
II.Constellation Vision system. Комплектация стандарт:
1.Консоль.
2.Лампа консоли.
3.Футляр для лампы консоли.
4.Антенна для передачи информации на принтер.
5.Руководство по эксплуатации.
6.Сканер штрих кодов.
7.Пульт дистанционного управления.
8.Держатель сканера.
9.Чехол консоли.
10.Шланг.
11.Держатель для инфузионного раствора.
12.Педаль управления.
13.Стойка-держатель.
14.Инструментальный столик.
15.Балласт.
16.Тележка-подставка.
17.Система для подключения газа.
18.Регулятор газового баллона ISPAN перфторпропан.
19.Регулятор газового баллона ISPAN серы гексафторид.
20.Чехол подставки.
21.Модуль освещения.
22.Лампа модуля освещения.
23.Футляр для лампы модуля освещения.
III. Constellation Vision system. Комплектация эконом:
1.Консоль.
2.Лампа консоли.
3.Футляр для лампы консоли.
4.Антенна для передачи информации на принтер.
5.Руководство по эксплуатации.
6.Сканер штрих кодов.
7.Пульт дистанционного управления.
8.Держатель сканера.
9.Чехол консоли.
10.Шланг.
11.Держатель для инфузионного раствора.
12.Педаль управления.
IV. Принадлежности:
1.Ультразвуковая рукоятка фрагматом.
2.Ультразвуковая рукоятка.
3.Рукоятка для установки пневматических инструментов.
4.Система для введения газов: шприц с тюбингом и микрофильтром.
5.Чехол для инструментального столика.
6.Мешок для сбора жидкости.
7.Набор для витректомии I:
— Зонд для витректомии.
— Кассета с мешком для сбора жидкости.
— Эндоосветитель.
— Инфузионная канюля.
— Комплект заглушек.
— Тюбинг для введения стерильного воздуха.
— Инфузионный тюбинг.
— Аспирационный тюбинг.
— Стерильная салфетка на монитор.
— Трехпозиционный переключатель.
— Шприц.
— Комплект: трокары с канюлями.
8.Набор для витректомии II:
— Зонд для витректомии.
— Кассета с мешком для сбора жидкости.
— Эндоосветитель.
— Инфузионная канюля.
— Комлект заглушек.
— Тюбинг для введения стерильного воздуха.
— Инфузионный тюбинг.
— Аспирационный тюбинг.
— Стерильная салфетка на монитор.
— Трехпозиционный переключатель.
— Шприц.
— Нож для склеротомии.
9.Набор для витректомии и факоэмульсификации I:
— Зонд для витректомии.
— Кассета с мешком для сбора жидкости.
— Эндоосветитель.
— Инфузионная канюля.
— Комплект заглушек.
— Тюбинг для введения стерильного воздуха.
— Инфузионный тюбинг.
— Ирригационно-аспирационный тюбинг.
— Стерильная салфетка на монитор.
— Трехпозиционный переключатель.
— Шприц.
— Тест-камера.
— Ключ для ирригационно-аспирационной рукоятки.
— Ирригационный рукав.
— Комплект трокары с канюлями.
10.Набор для витректомии и факоэмульсификации II:
— Зонд для витректомии.
— Кассета с мешком для сбора жидкости.
— Эндоосветитель.
— Инфузионная канюля.
— Комплект заглушек.
— Тюбинг для введения стерильного воздуха.
— Инфузионный тюбинг.
— Ирригационно-аспирационный тюбинг.
— Стерильная салфетка на монитор.
— Трехпозиционный переключатель.
— Шприц.
— Тест-камера.
— Ключ для ирригационно-аспирационной рукоятки.
— Ирригационный рукав.
— Комплект трокары с канюлями.
— Аспирационная/экструзионная линия.
11.Набор для витректомии и факоэмульсификации III:
— Зонд для витректомии.
— Кассета с мешком для сбора жидкости.
— Эндоосветитель.
— Инфузионная канюля.
— Комплект заглушек.
— Тюбинг для введения стерильного воздуха.
— Инфузионный тюбинг.
— Ирригационно-аспирационный тюбинг.
— Стерильная салфетка на монитор.
— Трехпозиционный переключатель.
— Шприц.
— Тест-камера.
— Ключ для ирригационно-аспирационной рукоятки.
— Ирригационный рукав.
— Аспирационная/экструзионная линия.
— Нож для склеротомии.
12.Набор для факоэмульсификации:
— Кассета с мешком для сбора жидкости.
— Комплект тюбингов для ирригации и аспирации.
— Тест-камера.
— Ключ для ирригационно-аспирационной рукоятки.
— Ирригационный рукав.
— Стерильная салфетка на монитор.
13.Наконечник для витректомии.
14.Инфузионный тюбинг с автоклапаном.
15.Аспирационный тюбинг.
16.Набор аксессуаров для фрагматома:
— Игла для фрагматома.
— Мандрен для прочищения иглы.
17.Ирригационно-аспирационный тюбинг.
18.Тюбинг для введения стерильного воздуха.
19.Набор для введения вязких жидкостей:
— Адаптер для подключения шприца.
— Шприц.
— Блокатор шприца.
— Канюли для введения.
— Заглушка-толкатель.
20.Пневматические ножницы.
21.Тюбинг для пневматических ножниц.
22.Тюбинг коаксиальный для витректомического наконечника.
23.Эндоокулярный световод.
24.Эндоокулярный световод с ирригацией.
25.Эндоокулярный лазерный зонд.
26.Эндоокулярный лазерный зонд с иллюминацией.
27.Эндоокулярный лазерный зонд с аспирацией.
28.Многофункциональный столик для инструментов:
— Стойка-держатель.
— Инструментальный столик.
— Балласт.
29.Мобильная подставка для системы:
— Тележка-подставка.
— Система для подключения газа.
— Регулятор газового баллона ISPAN перфторпропан.
— Регулятор газового баллона ISPAN серы гексафторид.
— Чехол подставки.
30.Лазерный модуль для системы:
— Лазер.
— Педаль управления лазером.
— Задняя панель для тележки-подставки.
— Интерфейс для подключения лазера.
— Лазерный блокиратор с ключами.
— Инструкция по подключению лазера.
31.Осветительный модуль:
— Модуль освещения.
— Лампа модуля освещения.
— Футляр для лампы модуля освещения.
32.Инструкция по применению.
33.Запасная лампа для осветительного модуля:
— Запасная лампа модуля освещения.
— Футляр для запасной лампы модуля освещения.
34.Флеш карта.
35.Беспроводной контролер функций управления.
36.Ножное устройство управления.
37.Газ ISPAN перфторпропан.
38.Газ ISPAN серы гексафторид.
39.Регулятор газового баллона ISPAN перфторпропан.
40.Регулятор газового баллона ISPAN серы гексафторид.
41.Прокладки для баллонов.
42.Подставка для баллонов.
43.Беспроводной принтер.
44.Устройство для видеозаписи.
45.Налобный офтальмоскоп.
46.Педаль управления лазером.
47.Аварийный выключатель лазера.
48.Лазерный офтальмоскоп.
49.Лазерный фильтр для операционного микроскопа.
50.Лазерный фильтр для щелевой лампы.
Подробнее
Развернуть
Система офтальмологическая хирургическая CONSTELLATION® VISION SYSTEM с принадлежностями (см. Приложение на 8 листах)I. Constellation Vision system. Комплектация люкс:
1.Консоль.
2.Лампа консоли.
3.Футляр для лампы консоли.
4.Антенна для передачи информации на принтер.
5.Руководство по эксплуатации.
6.Сканер штрих кодов.
7.Пульт дистанционного управления.
8.Держатель сканера.
9.Чехол консоли.
10.Шланг.
11.Держатель для инфузионного раствора.
12.Педаль управления.
13.Стойка-держатель.
14.Инструментальный столик.
15.Балласт.
16.Тележка-подставка.
17.Система для подключения газа.
18.Регулятор газового баллона ISPAN перфторпропан.
19.Регулятор газового баллона ISPAN серы гексафторид.
20.Чехол подставки.
21.Модуль освещения.
22.Лампа модуля освещения.
23.Футляр для лампы модуля освещения.
24.Лазер.
25.Педаль управления лазером.
26.Задняя панель для тележки-подставки.
27.Интерфейс для подключения лазера.
28.Лазерный блокиратор с ключами.
29.Инструкция по подключению лазера.
II.Constellation Vision system. Комплектация стандарт:
1.Консоль.
2.Лампа консоли.
3.Футляр для лампы консоли.
4.Антенна для передачи информации на принтер.
5.Руководство по эксплуатации.
6.Сканер штрих кодов.
7.Пульт дистанционного управления.
8.Держатель сканера.
9.Чехол консоли.
10.Шланг.
11.Держатель для инфузионного раствора.
12.Педаль управления.
13.Стойка-держатель.
14.Инструментальный столик.
15.Балласт.
16.Тележка-подставка.
17.Система для подключения газа.
18.Регулятор газового баллона ISPAN перфторпропан.
19.Регулятор газового баллона ISPAN серы гексафторид.
20.Чехол подставки.
21.Модуль освещения.
22.Лампа модуля освещения.
23.Футляр для лампы модуля освещения.
III. Constellation Vision system. Комплектация эконом:
1.Консоль.
2.Лампа консоли.
3.Футляр для лампы консоли.
4.Антенна для передачи информации на принтер.
5.Руководство по эксплуатации.
6.Сканер штрих кодов.
7.Пульт дистанционного управления.
8.Держатель сканера.
9.Чехол консоли.
10.Шланг.
11.Держатель для инфузионного раствора.
12.Педаль управления.
IV. Принадлежности:
1.Ультразвуковая рукоятка фрагматом.
2.Ультразвуковая рукоятка.
3.Рукоятка для установки пневматических инструментов.
4.Система для введения газов: шприц с тюбингом и микрофильтром.
5.Чехол для инструментального столика.
6.Мешок для сбора жидкости.
7.Набор для витректомии I:
— Зонд для витректомии.
— Кассета с мешком для сбора жидкости.
— Эндоосветитель.
— Инфузионная канюля.
— Комплект заглушек.
— Тюбинг для введения стерильного воздуха.
— Инфузионный тюбинг.
— Аспирационный тюбинг.
— Стерильная салфетка на монитор.
— Трехпозиционный переключатель.
— Шприц.
— Комплект: трокары с канюлями.
8.Набор для витректомии II:
— Зонд для витректомии.
— Кассета с мешком для сбора жидкости.
— Эндоосветитель.
— Инфузионная канюля.
— Комлект заглушек.
— Тюбинг для введения стерильного воздуха.
— Инфузионный тюбинг.
— Аспирационный тюбинг.
— Стерильная салфетка на монитор.
— Трехпозиционный переключатель.
— Шприц.
— Нож для склеротомии.
9.Набор для витректомии и факоэмульсификации I:
— Зонд для витректомии.
— Кассета с мешком для сбора жидкости.
— Эндоосветитель.
— Инфузионная канюля.
— Комплект заглушек.
— Тюбинг для введения стерильного воздуха.
— Инфузионный тюбинг.
— Ирригационно-аспирационный тюбинг.
— Стерильная салфетка на монитор.
— Трехпозиционный переключатель.
— Шприц.
— Тест-камера.
— Ключ для ирригационно-аспирационной рукоятки.
— Ирригационный рукав.
— Комплект трокары с канюлями.
10.Набор для витректомии и факоэмульсификации II:
— Зонд для витректомии.
— Кассета с мешком для сбора жидкости.
— Эндоосветитель.
— Инфузионная канюля.
— Комплект заглушек.
— Тюбинг для введения стерильного воздуха.
— Инфузионный тюбинг.
— Ирригационно-аспирационный тюбинг.
— Стерильная салфетка на монитор.
— Трехпозиционный переключатель.
— Шприц.
— Тест-камера.
— Ключ для ирригационно-аспирационной рукоятки.
— Ирригационный рукав.
— Комплект трокары с канюлями.
— Аспирационная/экструзионная линия.
11.Набор для витректомии и факоэмульсификации III:
— Зонд для витректомии.
— Кассета с мешком для сбора жидкости.
— Эндоосветитель.
— Инфузионная канюля.
— Комплект заглушек.
— Тюбинг для введения стерильного воздуха.
— Инфузионный тюбинг.
— Ирригационно-аспирационный тюбинг.
— Стерильная салфетка на монитор.
— Трехпозиционный переключатель.
— Шприц.
— Тест-камера.
— Ключ для ирригационно-аспирационной рукоятки.
— Ирригационный рукав.
— Аспирационная/экструзионная линия.
— Нож для склеротомии.
12.Набор для факоэмульсификации:
— Кассета с мешком для сбора жидкости.
— Комплект тюбингов для ирригации и аспирации.
— Тест-камера.
— Ключ для ирригационно-аспирационной рукоятки.
— Ирригационный рукав.
— Стерильная салфетка на монитор.
13.Наконечник для витректомии.
14.Инфузионный тюбинг с автоклапаном.
15.Аспирационный тюбинг.
16.Набор аксессуаров для фрагматома:
— Игла для фрагматома.
— Мандрен для прочищения иглы.
17.Ирригационно-аспирационный тюбинг.
18.Тюбинг для введения стерильного воздуха.
19.Набор для введения вязких жидкостей:
— Адаптер для подключения шприца.
— Шприц.
— Блокатор шприца.
— Канюли для введения.
— Заглушка-толкатель.
20.Пневматические ножницы.
21.Тюбинг для пневматических ножниц.
22.Тюбинг коаксиальный для витректомического наконечника.
23.Эндоокулярный световод.
24.Эндоокулярный световод с ирригацией.
25.Эндоокулярный лазерный зонд.
26.Эндоокулярный лазерный зонд с иллюминацией.
27.Эндоокулярный лазерный зонд с аспирацией.
28.Многофункциональный столик для инструментов:
— Стойка-держатель.
— Инструментальный столик.
— Балласт.
29.Мобильная подставка для системы:
— Тележка-подставка.
— Система для подключения газа.
— Регулятор газового баллона ISPAN перфторпропан.
— Регулятор газового баллона ISPAN серы гексафторид.
— Чехол подставки.
30.Лазерный модуль для системы:
— Лазер.
— Педаль управления лазером.
— Задняя панель для тележки-подставки.
— Интерфейс для подключения лазера.
— Лазерный блокиратор с ключами.
— Инструкция по подключению лазера.
31.Осветительный модуль:
— Модуль освещения.
— Лампа модуля освещения.
— Футляр для лампы модуля освещения.
32.Инструкция по применению.
33.Запасная лампа для осветительного модуля:
— Запасная лампа модуля освещения.
— Футляр для запасной лампы модуля освещения.
34.Флеш карта.
35.Беспроводной контролер функций управления.
36.Ножное устройство управления.
37.Газ ISPAN перфторпропан.
38.Газ ISPAN серы гексафторид.
39.Регулятор газового баллона ISPAN перфторпропан.
40.Регулятор газового баллона ISPAN серы гексафторид.
41.Прокладки для баллонов.
42.Подставка для баллонов.
43.Беспроводной принтер.
44.Устройство для видеозаписи.
45.Налобный офтальмоскоп.
46.Педаль управления лазером.
47.Аварийный выключатель лазера.
48.Лазерный офтальмоскоп.
49.Лазерный фильтр для операционного микроскопа.
50.Лазерный фильтр для щелевой лампы.
Подробнее
Развернуть
Система офтальмологическая хирургическая Constellation® Vision System с принадлежностями
I. Constellation Vision system. Комплектация люкс:
1.Консоль.
2.Лампа консоли.
3.Футляр для лампы консоли.
4.Антенна для передачи информации на принтер.
5.Руководство по эксплуатации.
6.Сканер штрих кодов.
7.Пульт дистанционного управления.
8.Держатель сканера.
9.Чехол консоли.
10.Шланг.
11.Держатель для инфузионного раствора.
12.Педаль управления.
13.Стойка-держатель.
14.Инструментальный столик.
15.Балласт.
16.Тележка-подставка.
17.Система для подключения газа.
18.Регулятор газового баллона ISPAN перфторпропан.
19.Регулятор газового баллона ISPAN серы гексафторид.
20.Чехол подставки.
21.Модуль освещения.
22.Лампа модуля освещения.
23.Футляр для лампы модуля освещения.
24.Лазер.
25.Педаль управления лазером.
26.Задняя панель для тележки-подставки.
27.Интерфейс для подключения лазера.
28.Лазерный блокиратор с ключами.
29.Инструкция по подключению лазера.
II.Constellation Vision system. Комплектация стандарт:
1.Консоль.
2.Лампа консоли.
3.Футляр для лампы консоли.
4.Антенна для передачи информации на принтер.
5.Руководство по эксплуатации.
6.Сканер штрих кодов.
7.Пульт дистанционного управления.
8.Держатель сканера.
9.Чехол консоли.
10.Шланг.
11.Держатель для инфузионного раствора.
12.Педаль управления.
13.Стойка-держатель.
14.Инструментальный столик.
15.Балласт.
16.Тележка-подставка.
17.Система для подключения газа.
18.Регулятор газового баллона ISPAN перфторпропан.
19.Регулятор газового баллона ISPAN серы гексафторид.
20.Чехол подставки.
21.Модуль освещения.
22.Лампа модуля освещения.
23.Футляр для лампы модуля освещения.
III. Constellation Vision system. Комплектация эконом:
1.Консоль.
2.Лампа консоли.
3.Футляр для лампы консоли.
4.Антенна для передачи информации на принтер.
5.Руководство по эксплуатации.
6.Сканер штрих кодов.
7.Пульт дистанционного управления.
8.Держатель сканера.
9.Чехол консоли.
10.Шланг.
11.Держатель для инфузионного раствора.
12.Педаль управления.
Принадлежности:
1.Ультразвуковая рукоятка фрагматом.
2.Ультразвуковая рукоятка.
3.Рукоятка для установки пневматических инструментов.
4.Система для введения газов: шприц с тюбингом и микрофильтром.
5.Чехол для инструментального столика.
6.Мешок для сбора жидкости.
7.Набор для витректомии I:
- Зонд для витректомии.
- Кассета с мешком для сбора жидкости.
- Эндоосветитель.
- Инфузионная канюля.
- Комплект заглушек.
- Тюбинг для введения стерильного воздуха.
- Инфузионный тюбинг.
- Аспирационный тюбинг.
- Стерильная салфетка на монитор.
- Трехпозиционный переключатель.
- Шприц.
- Комплект:
- трокары с канюлями.
8.Набор для витректомии II:
- Зонд для витректомии.
- Кассета с мешком для сбора жидкости.
- Эндоосветитель.
- Инфузионная канюля.
- Комлект заглушек.
- Тюбинг для введения стерильного воздуха.
- Инфузионный тюбинг.
- Аспирационный тюбинг.
- Стерильная салфетка на монитор.
- Трехпозиционный переключатель.
- Шприц.
- Нож для склеротомии.
9.Набор для витректомии и факоэмульсификации I:
- Зонд для витректомии.
- Кассета с мешком для сбора жидкости.
- Эндоосветитель.
- Инфузионная канюля.
- Комплект заглушек.
- Тюбинг для введения стерильного воздуха.
- Инфузионный тюбинг.
- Ирригационно-аспирационный тюбинг.
- Стерильная салфетка на монитор.
- Трехпозиционный переключатель.
- Шприц.
- Тест-камера.
- Ключ для ирригационно-аспирационной рукоятки.
- Ирригационный рукав.
- Комплект трокары с канюлями.
10.Набор для витректомии и факоэмульсификации II:
- Зонд для витректомии.
- Кассета с мешком для сбора жидкости.
- Эндоосветитель.
- Инфузионная канюля.
- Комплект заглушек.
- Тюбинг для введения стерильного воздуха.
- Инфузионный тюбинг.
- Ирригационно-аспирационный тюбинг.
- Стерильная салфетка на монитор.
- Трехпозиционный переключатель.
- Шприц.
- Тест-камера.
- Ключ для ирригационно-аспирационной рукоятки.
- Ирригационный рукав.
- Комплект трокары с канюлями.
- Аспирационная/экструзионная линия.
11.Набор для витректомии и факоэмульсификации III:
- Зонд для витректомии.
- Кассета с мешком для сбора жидкости.
- Эндоосветитель.
- Инфузионная канюля.
- Комплект заглушек.
- Тюбинг для введения стерильного воздуха.
- Инфузионный тюбинг.
- Ирригационно-аспирационный тюбинг.
- Стерильная салфетка на монитор.
- Трехпозиционный переключатель.
- Шприц.
- Тест-камера.
- Ключ для ирригационно-аспирационной рукоятки.
- Ирригационный рукав.
- Аспирационная/экструзионная линия.
- Нож для склеротомии.
12.Набор для факоэмульсификации:
- Кассета с мешком для сбора жидкости.
- Комплект тюбингов для ирригации и аспирации.
- Тест-камера.
- Ключ для ирригационно-аспирационной рукоятки.
- Ирригационный рукав.
- Стерильная салфетка на монитор.
13.Наконечник для витректомии.
14.Инфузионный тюбинг с автоклапаном.
15.Аспирационный тюбинг.
16.Набор аксессуаров для фрагматома:
- Игла для фрагматома.
- Мандрен для прочищения иглы.
17.Ирригационно-аспирационный тюбинг.
18.Тюбинг для введения стерильного воздуха.
19.Набор для введения вязких жидкостей:
- Адаптер для подключения шприца.
- Шприц.
- Блокатор шприца.
- Канюли для введения.
- Заглушка-толкатель.
20.Пневматические ножницы.
21.Тюбинг для пневматических ножниц.
22.Тюбинг коаксиальный для витректомического наконечника.
23.Эндоокулярный световод.
24.Эндоокулярный световод с ирригацией.
25.Эндоокулярный лазерный зонд.
26.Эндоокулярный лазерный зонд с иллюминацией.
27.Эндоокулярный лазерный зонд с аспирацией.
28.Многофункциональный столик для инструментов:
- Стойка-держатель.
- Инструментальный столик.
- Балласт.
29.Мобильная подставка для системы:
- Тележка-подставка.
- Система для подключения газа.
- Регулятор газового баллона ISPAN перфторпропан.
- Регулятор газового баллона ISPAN серы гексафторид.
- Чехол подставки.
30.Лазерный модуль для системы:
- Лазер.
- Педаль управления лазером.
- Задняя панель для тележки-подставки.
- Интерфейс для подключения лазера.
- Лазерный блокиратор с ключами.
- Инструкция по подключению лазера.
31.Осветительный модуль:
- Модуль освещения.
- Лампа модуля освещения.
- Футляр для лампы модуля освещения.
32.Инструкция по применению.
33.Запасная лампа для осветительного модуля:
- Запасная лампа модуля освещения.
- Футляр для запасной лампы модуля освещения.
34.Флеш карта.
35.Беспроводной контролер функций управления.
36.Ножное устройство управления.
37.Газ ISPAN перфторпропан.
38.Газ ISPAN серы гексафторид.
39.Регулятор газового баллона ISPAN перфторпропан.
40.Регулятор газового баллона ISPAN серы гексафторид.
41.Прокладки для баллонов.
42.Подставка для баллонов.
43.Беспроводной принтер.
44.Устройство для видеозаписи.
45.Налобный офтальмоскоп.
46.Педаль управления лазером.
47.Аварийный выключатель лазера.
48.Лазерный офтальмоскоп.
49.Лазерный фильтр для операционного микроскопа.
50.Лазерный фильтр для щелевой лампы.
Современная система для витректомии CONSTELLATION® Vision System объединяет функции для скоростного реза, контроля внутриглазного давления, освещения, лазерного излучения и других возможностей для удобства хирурга во время работы.
- Высокая скорость и контролируемый рабочий цикл манипуляций позволяют хирургам работать максимально близко к сетчатке.
- Контроль потока для инфузии вне зависимости от степени вакуума гарантирует точность для самых тонких процедур.
- Стабильность IOP осуществляется за счёт инфузии в реальном времени, а за его контроль отвечает специальный неинвазивный датчик.
- Запатентованная рукоятка OZil® Torsional Handpiece (торсионная рукоятка) обеспечивает великолепную точность работы.
- Система позволяет ввести индивидуальные настройки, а также автоматически распознаёт подключённые устройства.
- Обучающие видео программы для быстрых и безошибочных настроек.
Режимы работы
факоэмульсификация и трансциллиарная факофрагментация, пневматичекая витректомия, ввод вязких жидкостей, аспирация, микроножницы, биполярная и эндолазерная коагуляция
Скорость витректомии
до 5 000 резов/мин.
Инфузионное давление
0–120 мм.рт.ст
Частота биполярной коагуляции
1,5 Мгц
Частота факоэмульсификации
34–42 кГц
Ход ультразвуковой иглы
0–88,9±27 мкм
Скорость пневматических ножниц
0–450 резов/мин.
Рефлюкс
непрерывный и импульсный
Педаль управления
перепрограммируемая
Дисплей
17″ сенсорный, регулируемые угол наклона и яркость
THIS IS CONTROL.
THIS IS THE CONSTELLATION® VISION SYSTEM.
The gold standard in vitreoretinal surgery platforms.
IMPORTANT SAFETY INFORMATION PRODUCT VIDEOS
A HISTORY OF INNOVATION
A future that continues to redefine ophthalmic excellence.
Take command of vitreoretinal surgery
With the CONSTELLATION® Vision System, you can:
- Adjust flow independently of vacuum and cut rate with a surgeon-regulated duty cycle1
- Maintain a stable IOP with automatic and constant infusion-pressure monitoring2
- Effortlessly change voice-confirmed settings with a multifunctional footswitch control3
Integrate your OR
The CONSTELLATION® Vision System continuously evolves to power the newest vitreoretinal technologies from Alcon.

NGENUITY® 3D
VISUALIZATION SYSTEM
Real-time surgical feedback and immersive visualization on 1 screen


VEKTOR™ Articulating
Illuminated Laser Probe
Designed for unassisted scleral depression and built-in illumination4


FINESSE® SHARKSKIN®
ILM Forceps
A texturized surface and conforming grasping platform designed to improve grip during ILM peeling6,7
DELIVERING VITREORETINAL TECHNOLOGIES THAT WILL SHAPE TOMORROW
The constellation® Vision System defines ophthalmic innovation.
EXPERIENCE UNPRECEDENTED CONTROL AND PERFORMANCE
Integrates With The PUREPOINT® Laser
Features include voice confirmation, the multifunction footswitch, EnGauge® RFID, and dual laser attachment ports
Designed for surgeon control.
The CONSTELLATION® Vision System powers the HYPERVIT® Dual Blade Vitrectomy Probe and Advanced ULTRAVIT® Beveled High Speed Probe with unique dual-pneumatic technology.
Designed for stability.
With real-time infusion adjustments and Alcon’s proprietary Non-Invasive Flow Sensor technology, the CONSTELLATION® Vision System is designed to add a new measure of stability to vitrectomy procedures.
Designed for efficiency.
V-LOCITY® Efficiency Components—such as the 1-button Push Prime to speed up the priming sequence, an articulating arm tray for sterile setup, and EnGauge® RFID to automatically recognize each device you connect—help to increase productivity.

PRODUCT VIDEOS
Simultaneous Functionality in Challenging Cases
Vitreomacular Traction With Fibrotic Hyaloid
MIVS Important Product Information
Caution: Federal law restricts this device to sale by, or on the order of, a physician.
Indications for Use: The CONSTELLATION® Vision System is an ophthalmic microsurgical system that is indicated for both anterior segment (i.e., phacoemulsification and removal of cataracts) and posterior segment (i.e., vitreoretinal) ophthalmic surgery.
The ULTRAVIT® Vitrectomy Probe is indicated for vitreous cutting and aspiration, membrane cutting and aspiration, dissection of tissue and lens removal. The valved entry system is indicated for scleral incision, canulae for posterior instrument access and venting of valved cannulae. The infusion cannula is indicated for posterior segment infusion of liquid or gas.
Warnings and Precautions:
- The infusion cannula is contraindicated for use of oil infusion.
- Attach only Alcon supplied products to console and cassette luer fittings. Improper usage or assembly could result in a potentially hazardous condition for the patient. Mismatch of surgical components and use of settings not specifically adjusted for a particular combination of surgical components may affect system performance and create a patient hazard. Do not connect surgical components to the patient’s intravenous connections.
- Each surgical equipment/component combination may require specific surgical setting adjustments. Ensure that appropriate system settings are used with each product combination. Prior to initial use, contact your Alcon sales representative for in-service information.
- Care should be taken when inserting sharp instruments through the valve of the Valved Trocar Cannula. Cutting instrument such as vitreous cutters should not be actuated during insertion or removal to avoid cutting the valve membrane. Use the Valved Cannula Vent to vent fluids or gases as needed during injection of viscous oils or heavy liquids.
- Visually confirm that adequate air and liquid infusion flow occurs prior to attachment of infusion cannula to the eye.
- Ensure proper placement of trocar cannulas to prevent sub-retinal infusion.
- Leaking sclerotomies may lead to post operative hypotony.
- Vitreous traction has been known to create retinal tears and retinal detachments.
- Minimize light intensity and duration of exposure to the retina to reduce the risk of retinal photic injury.
ATTENTION: Please refer to the CONSTELLATION® Vision System Operators Manual for a complete listing of indications, warnings and precautions.
- Abulon Djk. Vitreous Flow Rates Through Dual Pneumatic Cutters: Effects Of Duty Cycle And Cut Rate. Clin Ophthalmol. 2015;9:253–261. Doi:10.2147/Opth.S71387.
- Riemann C, Zhou J, Buboltz Dc. Vitreous Cutter Velocities: Dual Pneumatic Drive Vs. Single Pneumatic Drive With Spring Return Probes. Poster Presented At: 2011 Annual Meeting Of The Association For Research In Vision And Ophthalmology; May 5, 2011; Fort Lauderdale, Fl.
- Houston Sks Iii. Get Behind The Wheel Of The Constellation. Retina Today. 2015;10(6):77–80. Http://Www.Retinatoday.Com/Pdfs/0915rt_cover_Combo.Pdf. Accessed March 21, 2019.
- Auld Jr, Farley Mh, Inventors; Alcon Research Ltd., Assignee. Multi-Fiber Flexible Surgical Probe. Us Patent Ca 2787024 A1. August 25, 2011.
- Alcon Data On File, 2018.
- Alcon Data On File, 2018.
©2021 Alcon Inc. 12/21 US-CON-2100031