Фармакологические (биологические) свойства и эффекты
Противококцидийное средство из группы ионофорных антибиотиков. Обладает выраженным кокцидиостатическим действием в отношении основных видов кокцидий, паразитирующих у птиц (Eimeria necatrix, Eimeria tenella, Eimeria acervulina, Eimeria brunetti, Eimeria maxima, Eimeria mivati) в стадии спорозоита, трофозонта и шизонта первого поколения.
Механизм действия мадурамицина заключается в избирательном нарушении транспорта ионов натрия и калия через биологические мембраны клетки паразита, что приводит к нарушению осмотического баланса и гибели кокцидий.
Мадурамицин аммония практически не всасывается в ЖКТ и оказывает свое действие на слизистой и подслизистой оболочках. Выводится из организма птиц, главным образом, в неизмененном виде с пометом в течение 2-3 дней.
Мало токсичен для теплокровных животных: LD50 для цыплят-бройлеров составляет более 2000 мг/кг массы тела. Не обладает местнорезорбтивным, местнораздражающим и сенсибилизирующим действием, но оказывает незначительное раздражающее действие на конъюнктиву
По степени воздействия на организм относится к умеренно опасным веществам (3 класс опасности ГОСТ 12.1.007-76).
Показания к применению препарата МАДУРАМИЦИН
Профилактика кокцидиоза у цыплят-бройлеров и ремонтного молодняка кур.
Порядок применения
Вводят в рацион птиц в дозе 500 г на 1 тонну корма, что соответствует 5 мг мадурамицина на 1 кг массы птицы, и применяют:
- цыплятам-бройлерам с первого дня жизни в течение всего периода выращивания и исключают из рациона за 5 дней до убоя;
- ремонтному молодняку кур с первого дня жизни до 16-недельного возраста.
Побочные эффекты
При применении в рекомендуемых дозах не вызывает у птиц побочных явлений и осложнений.
Противопоказания к применению препарата МАДУРАМИЦИН
- подтвержденная индивидуальная гиперчувствительность к мадурамицину;
- применение у лошадей (установлена высокая видоспецифическая токсичность мадурамицина для лошадей);
- применение курам-несушкам (т.к. мадурамицин накапливается в яйцах);
- применение одновременно с тиамулином, а также в течение 7 дней до и 7 дней после применения тиамулина;
- применение одновременно с другими противококцидийными средствами.
Особые указания и меры личной профилактики
Мадурамицин совместим с витаминами и известными кормовыми добавками, применяемыми в птицеводстве.
Убой птицы на мясо разрешается не ранее чем через 5 суток после окончания применения мадурамицина. Мясо птицы, вынужденно убитой до истечения указанного срока, может быть использовано для производства мясо-костной муки.
Все работы с мадурамицином необходимо проводить с использованием спецодежды и средств индивидуальной защиты (резиновые перчатки, защитные очки, респиратор). Во время работы запрещается пить, курить, и принимать пищу. По окончании работы следует тщательно вымыть с мылом лицо и руки, рот прополоскать водой.
-
Вы добавили товар в корзину:
ТИЛТИАМ (Россия), 5 л.
ТИЛТИАМ (Россия), 5 л.
Тилтиам применяют для профилактики и лечения птицы, больной бронхитом, микоплазмозом, эшерихиозом, пастереллёзом, а также при заболеваниях пищеварительного тракта и органов дыхания, вызванных микроорганизмами, чувствительными к тиамулину и тилозину.
Для лечения свиней, больных рожей, дизентерией, илеитом, лептоспирозом, листериозом, кампилобактериозом, колибактериозом, пастереллёзом, сальмонеллёзом, инфекционным гастроэнтеритом, спирохетным энтероколитом, атрофическим ринитом, энзоотической пневмонией, актинобациллярной плевропневмонией и микоплазматическим артритом, вызванных микроорганизмами, чувствительными к тиамулину и тилозину.
СТАЙЛАБ предлагает тест-системы для определения мадурамицина в кормах для животных, витаминных смесях и рисовых отрубях методом иммуноферментного анализа.
Мадурамицин – это ионофорный антибиотик и кокцидиостатик, входящий в состав таких препаратов, как Мадуро, Юмамицин, Мадикокс, Цигро и др. Он используется для профилактики и лечения эймериозов у птиц. Мадурамицин практически не растворяется в воде и, как большинство других ионофорных кокцидиостатиков, плохо всасывается в организмах животных, действуя, в основном, в кишечнике. Это вещество считается наиболее сильнодействующим и токсичным из всех ионофорных кокцидиостатиков.
Лошади плохо переносят воздействие мадурамицина. Даже в небольших дозах этот препарат может вызвать у них тяжелое отравление. Известно, что у коров и овец мадуромицин вызывает остановку сердца, кардиомиопатию, некроз мышц, у домашней птицы – диарею, анемию, лейкопению и лимфопению.
Зафиксированы случаи пищевых отравлений людей мадурамицином в результате употребления препаратов, содержащих его, в пищу. Для них были характерны полинейропатия (поражение нервов, приводящее к нарушению чувствительности, а в тяжелых случаях – к параличу конечностей), рабдомиолиз (некроз мышц, при котором мышечный белок миоглобин выделяется с мочой) и острая почечная недостаточность.
Нарушение сроков выдержки животных перед убоем может привести к тому, что мадурамицин сохранится в их тканях. Помимо этого, он может попадать в желток птичьих яиц, если несушки получают это вещество с кормом. Такое использование ветпрепаратов, содержащих мадурамицин, запрещено во многих странах.
В Российской Федерации и странах Таможенного Союза содержание мадурамицина во всех продуктах убоя ограничивает ТР ТС 034/2013 «О безопасности мяса и мясной продукции». С актуальными нормами можно ознакомиться на сайте compact24.com.
Для определения мадурамицина обычно используют хроматографические методы. Однако в качестве скринингового метода в настоящее время все чаще применяют тест-системы, основанные на методе иммуноферментного анализа (ИФА). Они позволяют за короткий промежуток времени проанализировать значительное количество проб. При этом тест-системы для анализа мадурамицина методом ИФА просты в использовании, удобны, высокочувствительны и точны.
Литература
- N Sharma, A Bhalla, S Varma, S Jain, S Singh.0 Toxicity of maduramicin. Emerg Med J 2005;22:880-882
- Varenina I, Bilandžić N, Cvetnić L, Kos B, Božić Đ, Solomun Kolanović B, Cvetnić Ž. Deposition and depletion of maduramicin residues in eggs after oral administration to laying hens determined by LC-MS. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2015;32(3):324-32.
- Chang KC, Su JJ, Cheng C. Development of online sampling and matrix reduction technique coupled liquid chromatography/ion trap mass spectrometry for determination maduramicin in chicken meat. Food Chem. 2013 Nov 15;141(2):1522-9.
- Clarke L, Moloney M, O’Mahony J, O’Kennedy R, Danaher M. Determination of 20 coccidiostats in milk, duck muscle and non-avian muscle tissue using UHPLC-MS/MS. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2013;30(6):958-69.
- Bodi D, Fry H, Schafft H, Lahrssen-Wiederholt M, Preiss-Weigert A. Carryover of maduramicin from feed containing cross-contamination levels into eggs of laying hens. J Agric Food Chem. 2012 Jul 18;60(28):6946-52.
Maduramicin ammonium is a polyether carboxylic ionophore agent that is authorized as a coccidiostat feed additive in the chicken and turkey for the control of E. adenoides, E. meleagrimitis, E.
From: Encyclopedia of Food Safety, 2014
Foods, Materials, Technologies and Risks
A. AnadónMR Martínez-Larrañaga, in Encyclopedia of Food Safety, 2014
Maduramicin
Maduramicin was approved for chickens in 1989. Maduramicin ammonium is a polyether carboxylic ionophore agent that is authorized as a coccidiostat feed additive in the chicken and turkey for the control of E. adenoides, E. meleagrimitis, E. gallopavonis, and E. dispersa. Maduramicin can inhibit the growth of Gram-positive microorganisms. Maduramicin and/or its metabolites are rapidly eliminated in chickens. Steady state is observed after 3 days in the excreta, but after 6 days in plasma. Maduramicin is the main compound excreted (26%). O-Demethylation represents the main metabolic pathway of maduramicin in chickens. Maduramicin is the marker residue in all tissues tested. Tissue residue kinetics of total residues and marker residue indicate a rapid decline of residues in all tissues.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780123786128002468
Role of pathology in diagnosis
Manu M. Sebastian, in Veterinary Toxicology, 2007
Ionophores
Monensin, lasalocid, salinomycin, narasin and maduramicin are carboxylic ionophores commonly used as anticoccidial drugs for poultry and as growth promotants for ruminants. Horses are the most susceptible species for ionophore toxicity and other domestic animals are also susceptible. Clinical pathological findings include elevated serum creatine phosphokinase (CPK), lactate dehydrogenase (LDH) and AST. The gross lesions include multifocal pale areas and hemorrhage in the pericardium and epicardium. Microscopic findings include degeneration and necrosis of cardiac and skeletal myocytes with fibrosis in chronic cases (Figure 90.2). Degeneration of the renal tubular epithelium and myoglobinuria are also reported in some cases (Novilla, 1992). Histopathological findings in horses are mostly associated with heart, commonly in the ventricles. The changes in bovines are also common in the heart but pigs have lesion in the skeletal muscles and atrium. Experimental studies in pigs dosed with toxic level of monensin showed pathological changes in the left atrium and consisted of extensive necrosis with contraction bands. The study indicates the selective injury of monensin in pigs to atrial myocardium (Muylle et al., 1981; Van Vleet and Ferrans, 1984).
FIGURE 90.2. Monensin toxicity, heart, bovine, cardiac myocytes show degeneration and some areas myocytes replaced and infiltrated by lymphocytes and histiocytes, H&E stain, 40×.
(Courtesy Dr. ME Hines, College of Veterinary Medicine, University of Georgia.)
Salinomycin has been reported to induce toxicity to the nervous system in cats. Cats which were exposed to feed material containing salinomycin had acute onset of lameness and paralysis of the hind limbs and forelimbs. Microscopic findings include neuropathy, myelin degeneration of the sensory and motor nerves (van der Linde-Sipman et al., 1999). Diagnosis is by case history, chemical analysis of the feed and pathological findings.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780123704672501875
Avian Toxicology
Arya Sobhakumari, … Snehal Tawde, in Veterinary Toxicology (Third Edition), 2018
Ionophores
Monensin, lasalocid, salinomycin, narasin, maduramicin, and semduramicin are some of the ionophore antibiotics used in poultry. Accidental or intentional off-label use has resulted in adverse reactions in adult poultry (laying hens), ostriches, and ornamental or game birds. In addition, interactions with other drugs used in target and nontarget species can occur. Various antibiotics have been reported to potentiate ionophore toxicity. The most frequently reported drug interaction is with the pleuromotilin derivative and tiamulin. This antibiotic interferes with the metabolic degradation of monensin in the liver, causing accumulation at toxic concentrations.
Excessive monensin exposure was reported in a 42-week broiler flock due to feed mixing error. There was high mortality and affected birds exhibited feed refusal, decreased water consumption, diarrhea and severe paralysis that ranged from abnormal gait to complete inability to move (Zavala et al., 2011). Another case report of monensin intoxication on a commercial ostrich farm in northern Greece described similar clinical signs along with elevated concentrations of serum aspartate aminotransferase, creatine kinase, and lactate dehydrogenase (Dedoussi et al., 2007). In layers, there will be a loss of egg production, hatchability, infertile eggs, early embryonic death, and weak ataxic chicks (Perelman and Smith, 1993). Reported cases of salinomycin intoxication in birds were associated with high mortality, signs of dyspnea, drowsiness, sternal recumbency with legs extended posteriorly, inability to stand, stiffness, and weakness (Andreasen and Schleifer, 1995). Gross postmortem findings may be absent or limited to hydropericardium, pale myocardium, hepatic congestion and enteritis. However, significant findings occur histologically and, include extensive fragmentation and necrosis of skeletal and myocardial muscle fibers (Dedoussi et al., 2007; Zavala et al., 2011).
Though avoidance of feed mixing errors and off-label use is the mainstay of prevention, results from few in vitro studies have been promising and show the protective effect of the herbal flavonolignan compound silybin from Silybum marinum against the toxicity of salinomycin, lasalocid, monensin and narasin in chicken hepatoma cell lines (Cybulski et al., 2015; Radko et al., 2013).
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780128114100000532
Ionophores
Meliton N. Novilla, in Veterinary Toxicology (Third Edition), 2018
Concluding Remarks and Future Directions
Seven ionophores—monensin, lasalocid, salinomycin, narasin, maduramicin, laidlomycin and semduramicin—are marketed globally for use as anticoccidial drugs for poultry and/or growth promotants in ruminants. Off-label usage of ionophore products is known to occur since other uses continue to be investigated and applied in many countries. It is likely that basic and applied research on these versatile compounds could lead in the future to product line extensions to other target species and potential development of novel therapeutics for unmet needs in veterinary and human medicine.
Generally, these feed additives have been found to be safe and effective in target animal species, but toxic syndromes have resulted from overdosage, misuse, and drug interaction. Among the domestic species, horses are the most sensitive to ionophore toxicoses, poultry the least sensitive, and cattle intermediate. However, even for the horse, there is a threshold level of exposure below which no adverse effects are observed. Consumption of complete feed containing the maximum approved use levels of monensin, lasalocid, or laidlomycin is harmless. Dose and time factors influence the severity and outcome of the toxic exposure. Results of controlled studies and confirmed field reports of toxicity indicate that the greatest risk of intoxication is upon initial exposure to ionophore-containing feed or supplement. Following sublethal exposure, consumption of culprit feed or supplement is negligible because of anorexia. Animals that die acutely after high levels of exposure often will have few or no lesions. Those that die later have profound striated (cardiac and/or skeletal) muscle lesions and changes secondary to CHF in some animals that survive the acute toxic episode.
Confirmatory diagnosis requires efficient laboratory assays to determine the identity and amounts of the ionophore involved and a thorough consideration of differential diagnosis. These cannot be overemphasized. There is no known antidote or specific treatment for ionophore toxicoses and treatment is largely supportive. Judicious use, avoidance of overdosing, and adherence to species recommendation will enhance livestock production and help prevent the occurrence of adverse effects associated with this class of compounds.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780128114100000787
Muscle and Tendon
Barry J. Cooper, Beth A. Valentine, in Jubb, Kennedy & Palmer’s Pathology of Domestic Animals: Volume 1 (Sixth Edition), 2016
Ionophore toxicosis
Ionophores used in agriculture are monensin, lasalocid, salinomycin, narasin, and maduramicin. Ionophores are compounds that alter membrane permeability to electrolytes by influencing transmembrane transport. In excess, all of these agents damage skeletal and cardiac muscle but horses are uniquely susceptible. Most reports of toxicosis involve monensin, an ionophore used widely for years.
Monensin, an antibiotic produced by the fermentation of Streptomyces cinnamonensis, has a growth-promoting effect in ruminants and is an efficient coccidiostat in birds and other animals. Monensin is produced commercially in very large quantities in North America and Europe, where it is added, as a concentrated premix, to pelleted or bulk feeds fed to cattle, sheep, and other ruminants. Toxicity develops when monensin is fed to monogastric animals, which have a much reduced tolerance for the drug, or when human or mechanical error leads to concentrations of monensin in the ration that are abnormally high for the species being fed. Toxic effects have been recorded in horses, donkeys, mules, zebras, cattle, sheep, dogs, wallabies, camels, blesbok, Stone sheep, turkeys, and chickens. Many episodes of monensin poisoning have been caused by mixing errors in packaged, pelleted, commercial animal feeds, either concentrates or final mix, which has put hundreds or thousands of animals at risk, sometimes over wide geographic areas. In North America alone, such mixing errors have been reported in horse, cattle, dog, and zoo feeds. Some indication of susceptibility of different species is provided by the estimated LD50 for different animals. Horses and other equids that are sensitive have an LD50 of 2-3 mg of monensin/kg body weight. LD50 values for other species are: dogs, 5-8 mg/kg; sheep and goats, 12-24 mg/kg; cattle, 50-80 mg/kg; and various types of poultry 90-200 mg/kg. Pigs, which may be given the drug for its coccidiostatic properties or be exposed by mistake, have an LD50 of 16-50 mg/kg body weight. The toxic effects of monensin or salinomycin are potentiated by the addition of tiamulin, triacetyloleandomycin, or sulfonamides to the ration, usually for therapeutic purposes.
Ingestion of maduramicin, an ionophore antibiotic used as a coccidiostat in poultry, has caused cardiotoxicity in cattle and sheep. Cases occurred in South Africa and Israel, where dried poultry litter was used as a source of protein for ruminants. The clinical and pathologic features of this toxicosis are similar to those of monensin cardiotoxicity.
When a single large toxic dose of an ionophore is fed to an animal, clinical signs of lethargy, stiffness, muscular weakness, and recumbency occur within 24 hours. Horses and other equids are likely, in the early stages, to show marked signs of colic, apprehension, shifting or fidgeting, sweating, myoglobinuria, and muscle tremors. Dogs show apprehension and progressive weakness. If sublethal doses are fed, the toxic effect will be cumulative, and the clinical onset may be delayed for 2-3 days to weeks depending on the total amount and the period over which it is fed, but the debility is likely to be more pronounced. Animals on low-level toxicity experiments often have delayed progression of the toxic signs because consumption of these feeds is reduced. These animals frequently scour and lose weight. At dose levels capable of inducing clinical signs of toxicity in a few days, many animals show evidence of progressive cardiac failure caused by a high incidence of myocardial lesions. Animals recovering from the acute disease may subsequently develop, within several months, signs of progressive cardiac insufficiency from myocardial fibrosis. Sometimes renal failure, in addition to poor growth or poor weight gain, occurs, although the signs referable to skeletal muscle injury may disappear.
Postmortem lesions of ionophore toxicity may be difficult to detect in acute cases dying within 24 hours. In horses, myocardial damage predominates, in sheep and swine the skeletal muscles are the main site of damage and myoglobinuria is generally present, and in cattle skeletal and cardiac muscle are about equally affected. Skeletal muscle may lack normal rigor, and ill-defined pale streaks may be visible in both myocardium and skeletal muscle. Later, the white streaking of affected skeletal muscles becomes more prominent. Hindlimb muscles may be the sites of major degenerative changes. Cases with terminal cardiac damage will have features of congestive heart failure including fluid accumulations in body cavities, pulmonary congestion and edema, and hepatic congestion.
Microscopic lesions of ionophore toxicity typically are multifocal monophasic necrosis by 48 hours after exposure and thus differ from the polyphasic lesions of nutritional myopathy. One of the earliest electron-microscopically visible lesions in muscle fibers is marked swelling and disintegration of mitochondria. Monensin is an ionophore that distorts membrane transport of sodium and potassium. This apparently leads to abnormalities of the electrolyte-modulated calcium gating mechanism and then mitochondrial failure, energy exhaustion, failure of calcium ion removal from the cytosol, and eventually myofibrillar hypercontraction and segmental degeneration. Both type 1 and type 2 fibers are involved with necrosis and macrophage infiltration. Satellite cell nuclei as well as endomysial cells apparently survive acute toxicity, and the early stages of regeneration are initiated during the first few days after exposure.
Myocardial lesions in monensin toxicity are not reparable and, particularly in a growing animal, the probability of lasting cardiac insufficiency is high.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780702053177000035
Cardiovascular System and Lymphatic Vessels1
Lisa M. Miller, Arnon Gal, in Pathologic Basis of Veterinary Disease (Sixth Edition), 2017
Toxicities
-
Cobalt, catecholamines, vasodilator antihypertensive drugs, methylxanthines (theobromine, theophylline, caffeine), ionophores (monensin, lasalocid, salinomycin, maduramicin, narasin), vitamin D and calcinogenic plants (Cestrum diurnum, Trisetum flavescens, Solanum malacoxylon, Solanum torvum), other poisonous plants (Acacia georginae, Gastrolobium spp., Oxylobium spp., Dichapetalum cymosum, Persea americana, Cassia occidentalis, Cassia obtusifolia, Karwinskia humboldtiana, Ateleia glazioviana, Eupatorium rugosum, Adonis aestivalis, Pachystigma pygmaeum, Fadogia homblei, Pavetta harborii, Tetrapterys multiglandulosa), blister beetles (Epicauta), high-erucic-acid rapeseed oil, brominated vegetable oils, gossypol, T-2 mycotoxin, sodium fluoracetate (compound 1080), selenium, uremia
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780323357753000102
Pathological biomarkers in toxicology
Meliton N. Novilla, … Stewart B. Jacobson, in Biomarkers in Toxicology, 2014
Toxic myopathy – ionophore toxic syndrome
Ionophore toxic syndromes have resulted from overdosage, misuse, and drug interactions of feed additives, including formulations with monensin, lasalocid, salinomycin, narasin, maduramicin, laidlomycin, or semduramicin. Target organs damaged by toxic doses of ionophores were identified to include the heart and skeletal muscles in all species studied (reviewed by Novilla, 2012). The most important change is a toxic myopathy characterized by focal areas of degeneration, necrosis, and repair in cardiac and skeletal muscles with a variable inflammatory component (Novilla and Folkerts, 1986; Van Vleet et al., 1991). The development of muscle lesions varies among domestic species. The heart is primarily affected in horses, skeletal muscle in pigs and dogs, and there is about equal tissue predilection in rats, chickens, and cattle. In addition, neurotoxic effects have been reported for lasalocid (Shlosberg et al., 1985; Safran et al., 1993), narasin (Novilla et al., 1994), and salinomycin (Van der Linde-Sipman et al., 1999). Neuropathic changes occurred in peripheral nerves and the spinal cord. Focal swelling, fragmentation, loss of axons, and formation of digestion chambers filled with macrophages were observed in both sensory and motor nerves, and there was vacuolation with swelling, degeneration, and fragmentation of myelin sheaths and axons in the spinal cord.
It is not easy to diagnose ionophore toxicoses. Clinical signs and muscle lesions of monensin toxicoses are not pathognomonic. In the absence of proof of a gross feed mixing error with monensin (usually >5X), the diagnostic pathologist must go through a process of exclusion of potential causes of the lesion. Confirmatory diagnosis requires laboratory assays to determine the identity and amounts of the ionophore involved or concurrent use of an incompatible drug.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780124046306000543
Detecting and controlling veterinary drug residues in poultry
V. Hagren, … T. Lovgren, in Food Safety Control in the Poultry Industry, 2005
Polyether ionophores
Ionophores are complex, high molecular weight molecules that are produced by various Streptomyces species. Ionophores (such as salinomycin, narasin, lasalocid, monensin, maduramicin and semduramicin) are active against Grampositive bacteria, mycobacteria, some fungi and certain parasites and coccidia. Ionophores have a rather unspecific mode of action: they form complexes with alkaline cations and disrupt the functioning of the cell membrane, which in turn influences the osmotic pressure of the cell. The withdrawal periods for ionophores vary between three and five days. Recently, the EU has established MRLs for lasalocid in poultry tissues (Anon., 2005). Because most ionophores do not have any chromophore structures, except for lasalocid, which has an intrinsically fluorescent chromophore, they cannot be detected with HPLC, without a derivatisation step. Ionophores are normally in the form of non-volatile sodium salts and therefore are not suitable for gas chromatographic analysis as such. Consequently, most methods rely on the use of LC-MS. An illustration of a mass spectrum is presented in Fig. 3.3.
Fig. 3.3. A typical example of a mass spectrum. Each peak represents a fragment of the molecule. The intensity of the peak is proportional to the relative abundance of the fragment.
The first report of an LC electrospray MS in ionophore analysis was published by Schneider et al. (1991). Another method, using HPLC–electro-spray MS for the detection of narasin, monensin, salinomycin and lasalocid was reported, with the advantage that only a single, quadruple MS system was needed (Harris et al., 1998). By adding ammonium acetate to the HPLC mobile phase, additional diagnostic ions could be produced to further confirm the identity of the analyte. However, no results of practical applications were presented in the study. Recently, two groups reported methods based on LC–electrospray tandem MS, using chicken eggs and liver as the sample matrices. One method was able to detect simultaneously narasin, monensin and salinomycin (Rosen, 2001) and the other lasalocid, narasin, monensin and salinomycin residues (Matabudul et al., 2002). The sample preparation in these studies was based on anhydrous sodium sulphate-acetonitrile extraction with SPE clean-up (Matabudul et al., 2002) or methanol extraction with automated SPE clean-up (Rosen, 2001). Both methods were very sensitive and had a high throughput. Therefore, they could be used for both screening and confirmatory purposes. Matabudul et al. (2000) also reported a two-tier testing system for lasalocid: an HPLC with fluorescence detection for screening and an LC–MS–MS for confirmation. A comprehensive review of the methods available for ionophore analysis was given by Elliott et al. (1998).
Several enzyme immunoassays for various ionophores have been described (Kennedy et al., 1995a,b, 1997; Muldoon et al., 1995; Shimer et al., 1996; Watanabe et al., 1998, 2001). In addition, immunoassays for monensin (Crooks et al., 1998b) and narasin/salinomycin (Peippo et al., 2004) that rely on the use of time-resolved fluorometry have been reported. The monensin immunoassay for poultry plasma samples described by Crooks et al. (1998b) utilised the dry chemistry assay concept, in which all the reagents needed for the assay were dry-coated in microtitre wells. Feeding experiments were also conducted to correlate the residue concentrations in plasma with those in liver. The narasin/salinomycin assay was used to screen residues from poultry muscle and eggs. The polyclonal antibody had a cross-reactivity of 100% for narasin and salinomycin, but less than 0.1% for other ionophores. The assay was very sensitive; the LOQ was 1.8 and 0.6 μg per kg for muscle and eggs, respectively.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B978185573954350003X
Comparative Pathology: Cardiovascular System
D.S. Russell, in Pathobiology of Human Disease, 2014
Ionophore Toxicosis
Dietary addition of ionophores has been used to enhance food conversion efficiency and manage coccidial disease in production animals. Ionophores used include monensin, lasalocid, salinomycin, maduramicin, and narasin. Accidental overdoses have been reported in various species including cattle, sheep, horses, and swine. Ionophores have also been used as an experimental model of toxic myocardial injury and irreversible myocardial dysfunction. Toxicity manifests to varying degrees in both the myocardium and skeletal muscle. Susceptibility differs among species, with the horse being particularly sensitive.
Monensin is a sodium (Na+)-selective carboxylic ionophore derived from the bacterium Streptomyces cinnamonensis. Lipid-soluble cation complexes traverse the cardiomyocyte membrane to increase the intracellular Na+ concentration. This change is accompanied by a corresponding increase in intracellular Ca2 + concentration, possibly via a transmembrane Na+/Ca2 + exchanger. When increases in intracellular Ca2 + exceed Ca2 +-sequestering capacity, numerous enzymes are activated – phospholipases, proteases, ATPases, and endonucleases – resulting in decreased cellular function or death. Other mechanisms that could contribute to cardiomyocyte death include catecholamine toxicity and peroxidative membrane injury. In support of the latter, supplementation of vitamin E and selenium in both cattle and swine can modify the course of disease and may offer a degree of protection when given as dietary supplements.
Ionophore toxicity is dose-dependent. Pathological findings are characterized by degeneration and necrosis of myocardium and skeletal muscle. Grossly, white linear streaks can be identified in striated muscles representing myofiber death and mineralization. Histological changes of lesions include swelling, hyalinization, vacuolar degeneration, and eventually loss of myofibers (Virtual Microscopy Slide 4 eSlide: VM00272). Acute ultrastructural changes include lipid deposition and mitochondrial swelling with accumulation of matrix densities. Consistent with intracellular Ca2 + accumulation, a short phase of hypercontraction may manifest morphologically as ‘contraction band’ necrosis. Macrophages infiltrate the necrotic myofiber tubes, followed by phagocytosis and activation of interstitial fibroblasts. In cattle, lesions are most apparent in the left ventricular myocardium. Selective atrial myocardial injury occurs in experimental models of porcine monensin intoxication.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780123864567034031
Approaches to Design and Synthesis of Antiparasitic Drugs
Satyavan Sharma, Nitya Anand, in Pharmacochemistry Library, 1997
3.6 Antibiotics
A number of antibiotics have been reported to possess antiprotozoal activities in humans and domestic animals. These include monensin (12) [100,101], salinomycin (13) [102,103], narasin (14) [104], maduramicin, (15) [105] lasalocid-A (16) [106], tetracycline (17a) [107,108], doxycycline (17b) [109], oxytetracycline (17c) [110], spiramycin (18) [111], amphotericin-B (19) [112], nystatin (20) [113], clindamycin (21) [114] and paromomycin (22) [115].
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/S0165720897800360
From Wikipedia, the free encyclopedia
 |
|
| Clinical data | |
|---|---|
| Other names | Maduramycin |
| AHFS/Drugs.com | International Drug Names |
| ATCvet code |
|
| Identifiers | |
| CAS Number |
|
| PubChem CID |
|
| ChemSpider |
|
| UNII |
|
| ChEMBL |
|
| Chemical and physical data | |
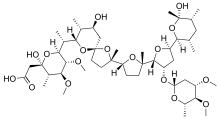
| Formula | C47H80O17 |
| Molar mass | 917.140 g·mol−1 |
| 3D model (JSmol) |
|
|
SMILES
|
Maduramicin (maduramycin) is an antiprotozoal agent used in veterinary medicine to prevent coccidiosis.[1][2] It is a natural chemical compound first isolated from the actinomycete Actinomadura rubra.[3]
References[edit]
- ^ Maduramicin Ammonium, Canadian Food Inspection Agency
- ^ McDougald LR, Fuller AL, Mathis GF, Wang GT (1990). «Efficacy of maduramicin ammonium against coccidiosis in turkeys under laboratory and floor-pen conditions». Avian Diseases. 34 (3): 634–638. doi:10.2307/1591256. JSTOR 1591256. PMID 2241692.
- ^ Fleck, W. F.; Strauss, D. G.; Meyer, Jutta; Porstendorfer, Gisela (1978). «Fermentation, isolation, and biological activity of maduramycin: a new antibiotic from Actinomadura rubra». Zeitschrift für Allgemeine Mikrobiologie. 18 (6): 389–98. doi:10.1002/jobm.3630180602. PMID 362738.




